Figure 3.
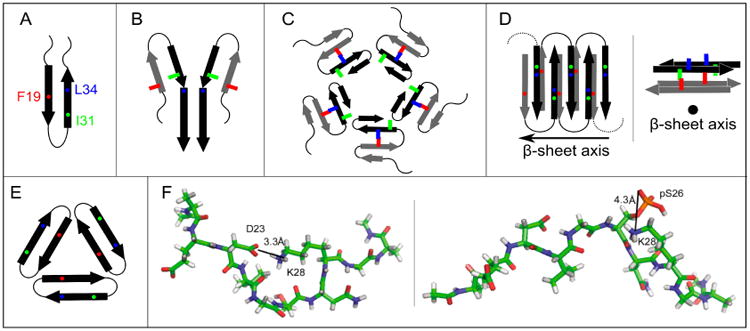
Proposed models for non-fibrillar Aβ aggregates. As in Figure 1, arrows, thin lines, and colored symbols represent β-strand and non-β-strand regions, and selected residues, respectively. (A) The antiparallel β-hairpin conformation predicted by Hoyer et al. for the monomer(114) and suggested by Scheidt et al. for protofibrils.(120,121) (B) The dimer structure proposed for preglobulomers by Yu et al.(123). (C) The disc-shaped pentamer model proposed by Ahmed et al.(124). (D) Two different views of the antiparallel β-sheet model for 150 kDa oligomers, reported by Tay et al.(111). (E) X-ray crystallographic structure of the trimer of the designed cyclic Aβ17-36 peptide.(127b) (F) Representative structures of highest populations in the MD ensembles of the Aβ21-30 WT peptide with the D23-K28 salt bridge (left) and the Aβ21-30 peptide with pS26 substitution (right).(47)
