Figure 5.
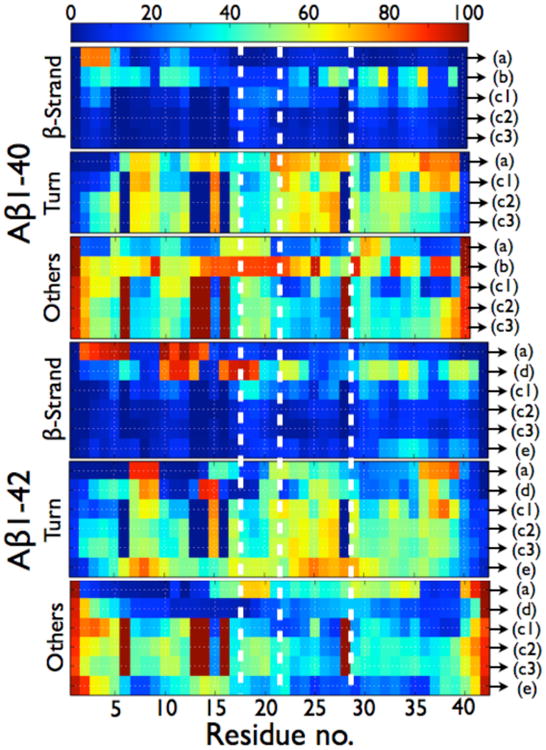
Per residue probabilities of β-strand, turn and others (coil and α-helix) for the Aβ1-40 and Aβ1-42 dimers as computed by different simulation protocols. As seen in Table 2, the total α-helix content amounts to less than 1.3% in Ref. 195, 202, 203 and 204 so others represent coil. In ref. 195, there is a total of 4.4% of α-helix in the dimers of Aβ1-42 and there is a probability of 27% for residues 23-28 to form α-helix. In Ref. 204, there is a total of 8.4% of α-helix in the dimers of Aβ1-42 and there is a probability of 45% for residues 13-17 to form a α-helix. (A) HT-REMD with the CG OPEP model and implicit solvent.(195) (B) united-atom CHARMM19 REMD with the SASA implicit solvent.(199) (C1) CG DMD with implicit solvent.(203) (C2) all-atom MD with OPLS-AA/SPCE.(204) (C3) all-atom MD with OPLS-AA/TIP3P.(204) (D) All-atom MC with implicit solvent.(181a) (E) all-atom REMD with OPLS-AA/TIP3P.(207) All secondary structure probabilities were computed using STRIDE; except for (b) for which the secondary structures are determined using information on the φ and Ψ dihedral angles only, without consideration of the H-bond network. As such, the β-sheet probabilities for (b) must be considered as extended conformations and no turn probability can be determined. The vertical dotted white lines delimit the four regions: N-terminal (residues 1-16), CHC (residues 17-21), loop region in the fibril (residues 22-28), and C-terminal (residues 29-40/42).
