Figure 7.
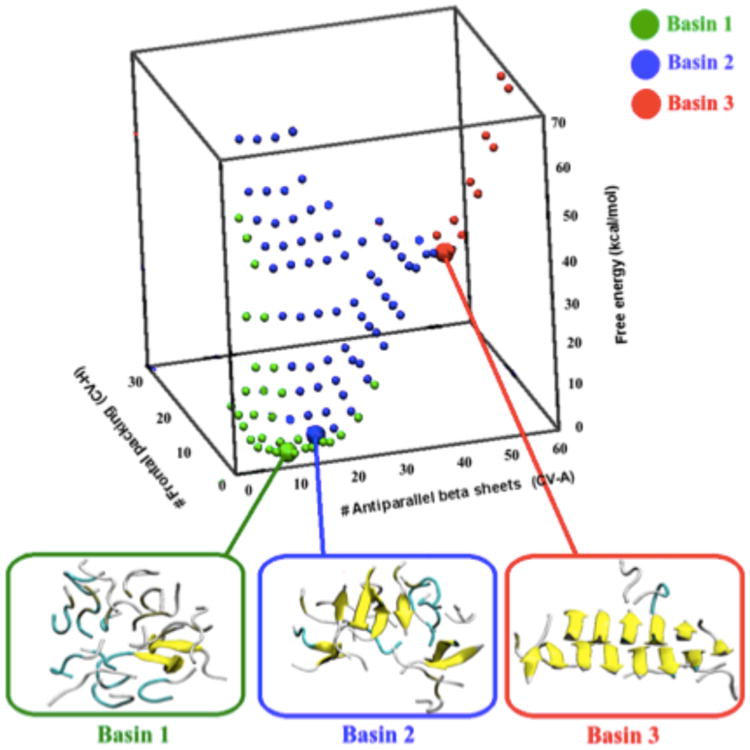
Free energy landscape of 18 Aβ35-40 peptides estimated by atomistic metadynamics simulations with explicit solvent as a function of the number of antiparallel β-sheets and the number of number of antiparallel frontal packing (different β-sheets facing each other).(178a) Basin 1 includes structures that are mainly disordered, with secondary structure elements formed only transiently. Basin 2 contains a much larger fraction of antiparallel β-sheets (up to 10–12 β-strands). The main characteristic of this basin is that the β-strands, although common, are not organized in a stable nucleus. These structures are only metastable, and can convert to the disordered melt in a few tenths of ns. Basin 3 includes instead structures with a specific inter-digitation of the side chains, which are stable at least on the ms time scale.
