Abstract
Aims
To investigate the relationship between pontine lesion characteristics on MRI and lower urinary tract symptoms (LUTS) in patients with multiple sclerosis (MS).
Methods
We performed a prospective cohort study of patients with MS and LUTS who were undergoing brain/spine MRI. Patients were administered the AUA Symptom Score (AUA-SS), Medical, Epidemiologic, and Social Aspects of Aging questionnaire (MESA), and Urinary Distress Inventory questionnaire (UDI-6), underwent Kurtzke Expanded Disability Status Scale (EDSS) scoring by a neurologist, and had their MRIs reviewed by a neuroradiologist. The relationships between symptom scores and lesion number, size, and location were analyzed.
Results
There were 42 patients that completed the study and 20 (48%) had one or more pontine lesions. Total AUA-SS and UDI-6 were related to multiple Short Form Health Survey (SF-36) scales and not EDSS scoring. Weak urinary stream measured on the AUA-SS (p=0.028), and urgency incontinence measured on the MESA questionnaire (p=0.034) were related to pontine lesion diameter. There was no difference in urinary symptoms according to the presence or absence of a pontine lesion, or according to lesion location within the pons.
Conclusions
Pontine lesion size appears to be related to lower urinary tract symptoms (weak stream and urgency incontinence) in patients with MS. Therefore, CNS lesion characteristics may be able to phenotype voiding symptoms in patients with MS.
Introduction
Multiple sclerosis (MS) is a disabling autoimmune inflammatory disease that affects millions of men and women worldwide(1). Patients with MS are often afflicted with lower urinary tract symptoms (LUTS), although the type and severity of their LUTS markedly vary (2, 3). The variation in LUTS experienced by patients with MS may be attributable to the variable locations of lesions in the central nervous system (CNS).
Previous studies have aimed to correlate the type and severity of voiding dysfunction in patients with MS with neuroanatomical lesion location. While results of these studies conflict (4-8), the pons appears to be a promising site in the central nervous system (CNS) where a correlation with the type of voiding dysfunction may be found. This is plausible because the pons contains the pontine micturition center (PMC), the nucleus responsible for supraspinal control of micturition. Prior studies have not examined the impact of lesion location or lesion size within the pons. Given the specific location of the PMC within the dorsal pons (9), LUTS may vary depending on the location/size of pontine lesions.
In this study, we investigated the relationship between pontine lesions and LUTS in patients with MS. We hypothesized that patients with pontine lesions would have more severe LUTS, and the severity of their LUTS would depend upon the location and size of the lesions within the pons.
Materials and Methods
We performed a prospective cohort study of adult patients with MS and lower urinary tract symptoms. Patients with MS were recruited from the Urologic and Neurologic outpatient offices. Patients were eligible to participate in the study if they were ≥ 18 years old, had a confirmed diagnosis of MS by a neurologist, complained of at least one lower urinary tract symptom, and had MRI imaging within the past year that was available for radiological review. Exclusion criteria for the study included: an MS relapse within the last 4 weeks, treatment with steroids or other drugs prescribed for a MS exacerbation within the last 4 weeks, treatment with a bladder medication or urologic intervention within the last 8 weeks, other neurologic disease, active or recent urinary tract infection, and other bladder disease.
Patients were administered the American Urological Association Symptom Score (AUA-SS), the Medical, Epidemiologic, and Social Aspects of Aging questionnaire (MESA), the Urinary Distress Inventory (UDI-6), and the Short Form Health Survey (SF-36). Patients also underwent neurologic examination, whereby the degree of their disability was measured by the Kurtzke Expanded Disability Status Scale (EDSS). The EDSS ranges from 0 (normal) to 10 (death due to MS) with a 0.5-step size. The functional systems included in the EDSS are pyramidal, cerebellar, brainstem, sensory, bowel and bladder, visual, and mental, and are graded from 0 (normal) to 5 (maximal impairment). The neurologist scoring the EDSS was blinded to the patient's urinary symptom scores and MRI findings.
Brain and spine MRIs were reviewed by a neuroradiologist. The presence of lesions in the pons, cerebellum, cerebrum, cervical spine, and thoracic spine were recorded. The presence of brain and/or spinal cord atrophy was noted as well. For patients with pontine lesions, the number of pontine lesions, size (diameter) of the largest pontine lesion, and location of the lesions within the pons was recorded. For the purposes of the study, the pons was divided into the ventral pons (tissue bulge anterior to the medulla) and the dorsal pons (pontine tissue above the medulla). Lesion activity (i.e., enhancement) was also recorded for each lesion. MRIs were performed with a 3T magnet. The neuroradiologists were blinded to the urinary and disability metrics.
Approval for this study was obtained by the Institutional Review Board at the University of ____. Spearman's rank correlation was used to calculate the correlation between the AUA-SS and the degree of disability, and a Bonferroni adjustment was applied. Student's t tests were used to compare the mean urinary symptoms scores between patients with and without pontine lesions. Linear regression was used to test the relationship between the AUA-SS and lesion location as well as urinary symptom scores and the size and number of pontine lesions. P-values <0.05 were considered statistically significant. Stata version 12.1 (College Station, Texas) was used for statistical analysis.
Results
42 patients that completed all questionnaires and had an MRI available for review were included in the study. 20 (48%) patients had one or more pontine lesions (Figure 1). Mean patient age was not different between patients with and without pontine lesions, and most patients had the relapsing and remitting type of multiple sclerosis (Table 1). Of patients with pontine lesions, 11 (55%) had a single pontine lesion, 5 (25%) had 2 lesions, and 4 (20%) had 3 or more pontine lesions. 12 (60%) patients had lesions in the ventral pons only, 2 (10%) had lesions in the dorsal pons only, and 6 (30%) had lesions in the ventral and dorsal pons. Mean pontine lesion size was 2.8mm (SD 0.51). There was no difference between patients with and without pontine lesions in the presence of a concomitant lesion in the cerebrum, cerebellum, or cord, or in the presence of cerebral/cord atrophy.
Figure 1.
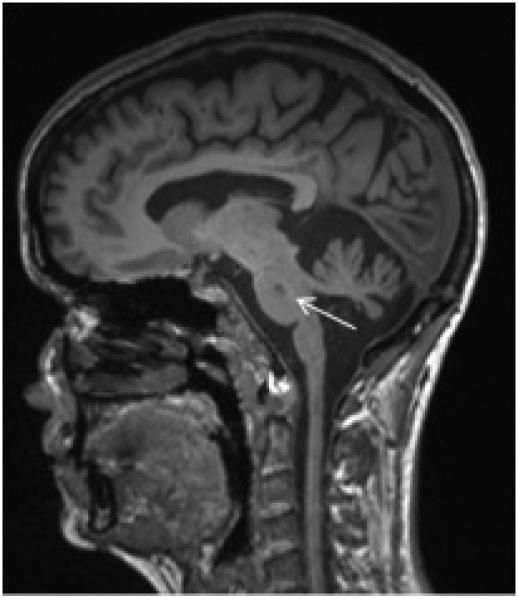
Sagittal T1 Brain MRI Demonstrating Pontine Lesion (arrow)
Table I.
Patient Demographics and Characteristics According to Pontine Lesion Group
| Parameter | Pons lesion (n=20) | No Pons lesion (n=22) | P-value |
|---|---|---|---|
| Age, mean (SD) | 48.7 (2.9) | 50.9 (2.8) | 0.585 |
| Gender, Number Female (%) | 18 (90%) | 20 (91%) | 0.659 |
| BMI, mean (SD) | 28.5 (2.0) | 27.1 (1.2) | 0.551 |
| Duration of MS, mean years (SD) | 14.3 (1.6) | 11.0 ( 1.6) | 0.16 |
| Type of MS, Number RRMS (%) | 13 (59%) | 14 (70%) | 0.461 |
| Time since bladder symptoms began, mean years (SD) | 12.2 (1.3) | 11.2 (1.3) | 0.576 |
| Pregnant at least once, Number (%) | 11 (61%) | 12 (60%) | 0.944 |
| Race, Number White/Caucasian (%) | 12 (60%) | 19 (90%) | 0.039 |
| EDSS score, mean (SD) | 4.5 (0.4) | 3.8 (0.4) | 0.253 |
| Cerebral Lesions, Number (%) | 20 (100%) | 21 (95%) | 0.335 |
| Cerebellar Lesions, Number (%) | 7 (35%) | 6 (28%) | 0.744 |
| Cervical Cord Lesions, Number (%) | 19(95%) | 17(77%) | 0.108 |
| Thoracic Cord Lesions, Number (%) | 18 (90%) | 17(77%) | 0.128 |
| Cerebral atrophy, Number (%) | 10 (50%) | 7 (32%) | 0.231 |
| Cord atrophy, Number (%) | 4 (20%) | 3 (13%) | 0.444 |
Moderate correlation existed between SF-36 scales and the AUA-SS, and UDI-6 (Table 2). For the AUA-SS and for the UDI-6, the correlation persisted between the general health and pain scales, respectively, after applying the Bonferroni adjustment such that worsening urinary symptom scores correlated with worsening general health and pain scale score. There was no relationship between the total EDSS physical disability score and the AUA-SS (data not shown).
Table II.
Correlation Between Urinary Symptoms and Disability
| AUA SS | UDI-6 | |||
|---|---|---|---|---|
| SF-36 Scale | Coefficient | P-value | Coefficient | P-value |
| Physical functioning | −0.06 | 0.683 | −0.08 | 0.620 |
| Role Limitations due to Physical Health | −0.23 | 0.138 | −0.40 | 0.009 |
| Role Limitations due emotional Problems | −0.40 | 0.008 | −0.23 | 0.143 |
| Energy/fatigue | −0.30 | 0.055 | −0.26 | 0.091 |
| Emotional well being | −0.42 | 0.006 | −0.41 | 0.007 |
| Social functioning | −0.18 | 0.246 | −0.27 | 0.085 |
| Pain | −0.31 | 0.049 | −0.49 | 0.001 |
| General Health | −0.48 | 0.001 | −0.29 | 0.058 |
There was no difference in the AUA-SS between patients with and without pontine lesions, although a difference in weak stream approached significance, Table 3. There was, however, a statistically significant relationship between the size (diameter) of the largest pontine lesion with weak urinary stream on the AUA-SS, as well as with urgency incontinence reported on the MESA questionnaire, Table 4. There was no difference in total AUA-SS between patients with and without ventral pontine lesions (17.8 versus 15.3, P=0.190).
Table III.
Urinary Symptoms According to Pontine Lesion Group
| Parameter | Pons Lesion (n=20) | No Pons Lesion (n=22) | P-value |
|---|---|---|---|
| Incomplete emptying (mean, SD) | 2.4 (1.9) | 2.0 (1.8) | 0.497 |
| Frequency (mean, SD) | 3.2 (1.7) | 2.87 (1.5) | 0.555 |
| Intermittency (mean, SD) | 1.7 (1.5) | 2.2 (1.9) | 0.325 |
| Urgency (mean, SD) | 2.7 (1.9) | 2.6 (1.7) | 0.908 |
| Weak stream (mean, SD) | 2.8 (1.7) | 1.8 (1.7) | 0.072 |
| Straining (mean, SD) | 2.4 (1.8) | 1.7 (1.7) | 0.253 |
| Nocturia (mean, SD) | 2.5 (1.7) | 2.2 (1.6) | 0.535 |
| Total (mean, SD) | 17.4 (6.1) | 15.5 (5.8) | 0.295 |
| Storage (mean, SD) | 8.4 (3.5) | 7.7 (2.6) | 0.483 |
| Voiding score (mean, SD) | 9.2 (4.6) | 7.7 (5.2) | 0.336 |
| QoL (mean, SD) | 3.5 (1.2) | 3.4 (1.3) | 0.820 |
| MESA Urge Subscale | 6.7 (5.2) | 4.9 (3.8) | 0.169 |
| MESA Stress Subscale | 8.3 (8.1) | 5.3 (6.2) | 0.152 |
| UDI-6 | 40.5 (23.6) | 32.5 (19.7) | 0.240 |
Table IV.
Relationship Between Urinary Symptoms and Pontine Lesion Size
| Parameter | Coefficient | 95% CI | P-value | ||
|---|---|---|---|---|---|
| Incomplete emptying (mean, SD) | 0.05 | −0.13 | - | 0.23 | 0.586 |
| Frequency (mean, SD) | 0.02 | −0.14 | - | 0.17 | 0.838 |
| Intermittency (mean, SD) | −0.06 | −0.22 | - | 0.11 | 0.511 |
| Urgency (mean, SD) | 0.06 | −0.11 | - | 0.24 | 0.455 |
| Weak stream (mean, SD) | 0.19 | 0.02 | - | 0.35 | 0.028 |
| Straining (mean, SD) | 0.12 | −0.05 | - | 0.29 | 0.157 |
| Nocturia (mean, SD) | 0.03 | −0.13 | - | 0.19 | 0.709 |
| Total (mean, SD) | 0.39 | −0.18 | - | 0.97 | 0.176 |
| Storage (mean, SD) | 0.11 | −0.19 | - | 0.41 | 0.464 |
| Voiding score (mean, SD) | 0.30 | −0.17 | - | 0.78 | 0.207 |
| QoL (mean, SD) | 0.04 | −0.08 | - | 0.17 | 0.509 |
| MESA Urge Subscale | 0.44 | 0.04 | - | 0.85 | 0.034 |
| MESA Stress Subscale | 0.15 | −0.19 | - | 1.21 | 0.150 |
| UDI-6 | 0.02 | −0.03 | - | 0.06 | 0.452 |
Discussion
We compared LUTS in patients with MS according to the presence and characteristics of pontine lesions with the objective of trying to explain the variability in LUTS experienced by these patients. As the pontine micturition center (Barrington's Nucleus) is vital to the coordination of the micturition reflex (by receiving neuronal input from the bladder via the periaqueductal grey and hypothalamus and sending fibers to the sacral cord)(9), one would expect that patients with pontine disease would have more severe LUTS. In this study, we found no difference in LUTS between patients with and without pontine lesions secondary to MS. Urinary symptoms were related to self-reported disability and the size of the largest pontine lesion, suggesting that pontine disease likely plays a role in the pathogenesis of LUTS. Specifically, patients with larger pontine lesions reported worse weak stream on the AUA-SS and urgency incontinence on the MESA, albeit the effect was small. Lesion location within the pons did not appear to affect LUTS.
Voiding dysfunction is common in patients with MS (3). Despite the well-known relationship between MS and voiding dysfunction (2), the relationship between the neuroanatomical lesion location and the type and severity of LUTS in these patients is unclear. Understanding this relationship is important in order to further elucidate the pathophysiological relationship between MS and LUTS. Additionally, as patients with MS have heterogeneous responses to treatments (10), understanding the connection between the anatomical lesion location and LUTS in these patients could lay the groundwork for future studies investigating whether neuroimaging characteristics can be predictive of the treatment outcomes.
Overall, while we found that LUTS were not related to the presence of pontine disease, weak stream and urgency incontinence were related to the size of pontine lesions. Therefore, the characteristics of an MS lesion may be more contributory to LUTS than its mere presence or absence. Prior studies that have examined the relationship between LUTS and brain MRI findings report conflicting results. Kim et al found that IPSS scores in patients with MS were not related to the total number of lesions on cranial MRIs; however, in this study the relationship of LUTS with pontine lesions was not investigated. (4). On the other hand, Araki et al found a relationship between the presence of a pontine lesion and detrusor hyporeflexia but not with patient reported IPSS score (6). Although Charil et al did not utilize a LUTS questionnaire for their study, the authors found a correlation between bowel and bladder scores on the EDSS with lesions in the pons(7).
Plausibly, larger pontine lesions are more likely to affect neural pathways involved in the micturition reflex. In addition to the PMC, other vital neuronal centers reside in the pons and may be affected by inflammation and/or demyelination as a result of MS. For instance, the pontine storage center, which stimulates external urethral sphincter contractions (via the Onuf`s nucleus) and inhibits detrusor contraction, is located ventrally and laterally to the PMC (11). Although the periaqueductal gray (PAG) is located in the midbrain, its fibers project to the PMC (12); the PAG is excitatory to the PMC and the micturition reflex (13). While we did not find a difference in LUTS according to lesion location, the nuclei involved in the micturition reflex cannot reliably be isolated on MRI. Knowing this, we still decided to divide the pons into ventral and dorsal areas, as this provided a practical way of assessing the impact of pontine lesion location on LUTS. Aside from affecting a specific pathway, pontine lesions may represent a marker of more extensive damage to other parts of the CNS in patients with MS, thus resulting in dyssynergia and weak stream.
A limitation to this study is that we were unable to study pontine lesions in isolation, as 93% of patients had concomitant spinal cord lesions, and 97% had concomitant cerebral lesions, thereby limiting our ability to assess their individual effects on LUTS. Another limitation to the study is the heterogeneity in our patient population with respect to type and severity of voiding symptoms, and we did not account for other pathologies which may have contributed to LUTS in these patients (e.g., diabetes). Furthermore, the small number of dorsal pons lesions in this study may have left us underpowered to detect a difference.
Future research investigating the relationship between radiographic evidence of MS disease and LUTS may focus on more advanced imaging techniques than standard MRI. Functional MRI, which measures areas of brain activity, may shed light on the pathogenesis of voiding dysfunction in patients with MS (14). While non-functional imaging can detect the presence and characteristics of a lesion, functional imaging can be used to investigate how a given lesion affects neural control of the lower urinary tract function. Aside from imaging, additional research studies may be aimed at investigating the role of urinary and serum biomarkers to assess and monitor the progression of LUTS in patients with MS (15).
Conclusion
In conclusion, while the mere presence of a lesion in the pons does not appear to affect LUTS in patients with MS, pontine lesion size was found to be related to weak urinary stream and urgency incontinence. Further research focused on more detailed characterization of MS lesions is needed to further delineate the pathogenesis of LUTS in patients with MS.
Acknowledgments
Funding NIH/NIDDK 1 P20 DK097819-01
Key of Definitions for Abbreviations
- MS
multiple sclerosis
- LUTS
lower urinary tract symptoms
- CNS
central nervous system
- PMC
pontine micturition center
- AUA-SS
American Urological Association Symptom Score
- MESA
Medical, Epidemiologic, and Social Aspects of Aging questionnaire
- UDI-6
Urinary Distress Inventory questionnaire
- SF-36
Short Form Health Survey
- EDSS
Kurtzke Expanded Disability Status Scale
References
- 1.Browne P, Chandraratna D, Angood C, Tremlett H, Baker C, Taylor BV, et al. Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology. 2014;83(11):1022–4. doi: 10.1212/WNL.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litwiller SE, Frohman EM, Zimmern PE. Multiple sclerosis and the urologist. J Urol. 1999;161(3):743–57. [PubMed] [Google Scholar]
- 3.Fowler C. The cause and management of bladder, sexual and bowel symptoms in multiple sclerosis. Baillieres Clin Neurol. 1997;6(3):447–66. [PubMed] [Google Scholar]
- 4.Kim YH, Goodman C, Omessi E, Rivera V, Kattan MW, Boone TB. The correlation of urodynamic findings with cranial magnetic resonance imaging findings in multiple sclerosis. J Urol. 1998;159(3):972–6. [PubMed] [Google Scholar]
- 5.Araki I, Matsui M, Ozawa K, Nishimura M, Kuno S, Saida T. Relationship between urinary symptoms and disease-related parameters in multiple sclerosis. J Neurol. 2002;249(8):1010–5. doi: 10.1007/s00415-002-0775-4. [DOI] [PubMed] [Google Scholar]
- 6.Araki I, Matsui M, Ozawa K, Takeda M, Kuno S. Relationship of bladder dysfunction to lesion site in multiple sclerosis. J Urol. 2003;169(4):1384–7. doi: 10.1097/01.ju.0000049644.27713.c8. [DOI] [PubMed] [Google Scholar]
- 7.Charil A, Zijdenbos AP, Taylor J, Boelman C, Worsley KJ, Evans AC, et al. Statistical mapping analysis of lesion location and neurological disability in multiple sclerosis: application to 452 patient data sets. Neuroimage. 2003;19(3):532–44. doi: 10.1016/s1053-8119(03)00117-4. [DOI] [PubMed] [Google Scholar]
- 8.Ukkonen M, Elovaara I, Dastidar P, Tammela T. Urodynamic findings in primary progressive multiple sclerosis are associated with increased volumes of plaques and atrophy in the central nervous system. Acta Neurol Scand. 2004;109(2):100–5. doi: 10.1034/j.1600-0404.2003.00184.x. [DOI] [PubMed] [Google Scholar]
- 9.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9(6):453–66. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicholas RS, Friede T, Hollis S, Young CA. Anticholinergics for urinary symptoms in multiple sclerosis. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD004193.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Blok B, Holstege G. The central control of micturition and continence: implications for urology. BJU Int. 1999;83(S2):1–6. doi: 10.1046/j.1464-410x.83.s2.2.x. [DOI] [PubMed] [Google Scholar]
- 12.Blok BF, Holstege G. Direct projections from the periaqueductal gray to the pontine micturition center (M-region). An anterograde and retrograde tracing study in the cat. Neurosci Lett. 1994;166(1):93–6. doi: 10.1016/0304-3940(94)90848-6. [DOI] [PubMed] [Google Scholar]
- 13.Holstege G, Mouton LJ. Central nervous system control of micturition. Int Rev Neurobiol. 2003;56:123–45. doi: 10.1016/s0074-7742(03)56004-4. [DOI] [PubMed] [Google Scholar]
- 14.Filippi M, Rocca MA. Present and future of fMRI in multiple sclerosis. Expert Rev Neurother. 2013;13(12s):27–31. doi: 10.1586/14737175.2013.865871. [DOI] [PubMed] [Google Scholar]
- 15.Cruz CD, Coelho A, Antunes-Lopes T, Cruz F. Biomarkers of spinal cord injury and ensuing bladder dysfunction. Adv Drug Deliv Rev. 2014 doi: 10.1016/j.addr.2014.11.007. [DOI] [PubMed] [Google Scholar]


