Figure 1. Signals that activate NF-κB and STAT1 enhance chemokine production within pancreatic islets that contribute to the inflammatory response influencing both insulin secretion and total β-cell mass.
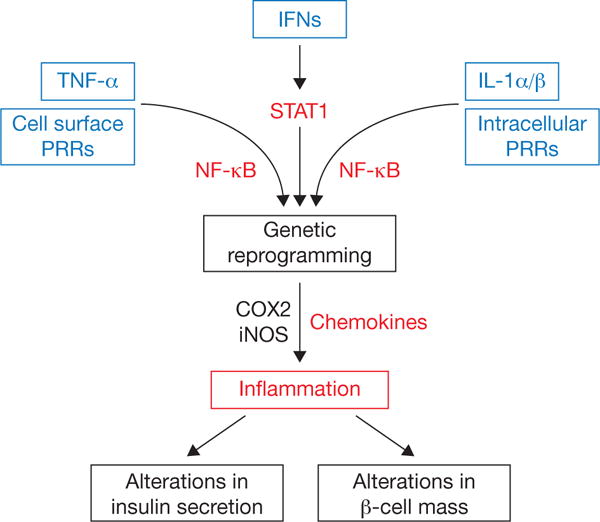
Many signals converge on the NF-κB and STAT1 pathways to coordinately reprogram beta cells at a transcriptional level, leading to inflammation-based changes in insulin secretion, β-cell mass, or both. PRRs can be either cell surface based, such as Toll-like receptor-2 and -4 or positioned intracellularly (e.g., Toll-like receptor-3, NOD1, NOD2, etc.). TNF-α and IL-1 signal through specific cell surface receptors (receptor not shown) and converge on the NF-κB pathway. The interferon family of cytokines signals through cell surface receptors that activate JAK-STAT pathways. Activation of COX2 produces prostaglandins, which may influence leukocyte activity. Cytokine-mediated increases in iNOS elevate intracellular production of nitric oxide, which acts as a rheostat for insulin secretion. COX2: cyclooxygenase-2; IFNs: interferons alpha, beta, and gamma; iNOS: inducible nitric oxide synthase; PRR: pattern recognition receptors.
