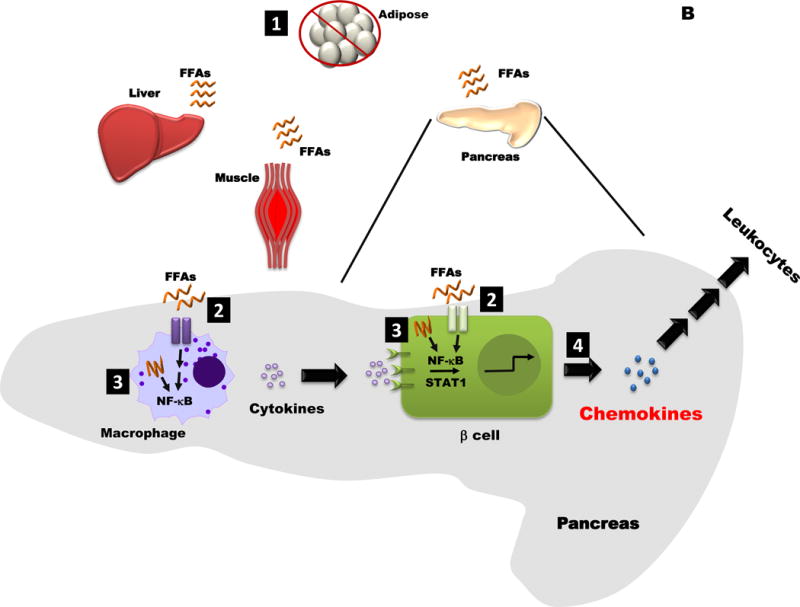
Figure 2. Conceptual Models for how Obesity may influence Islet Inflammation and Chemokine Production.
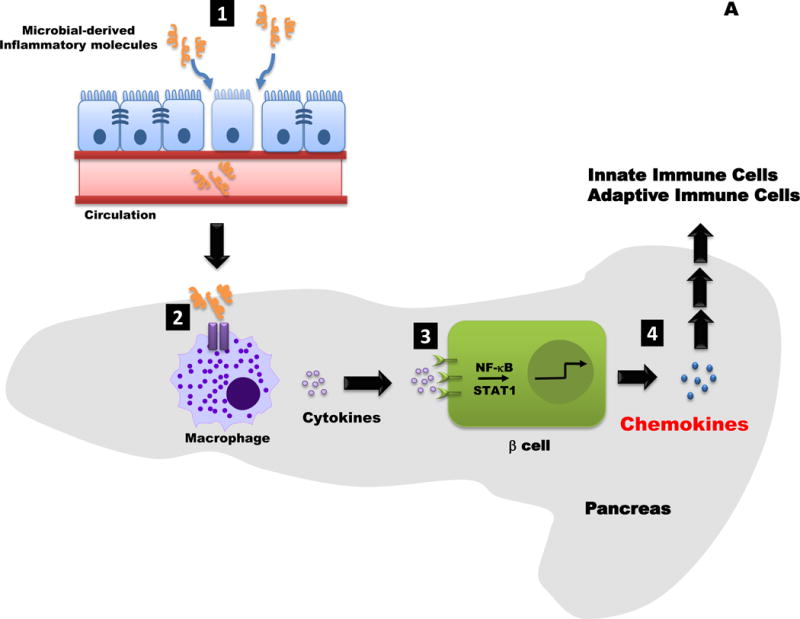
A. 1. Increased intestinal permeability allows microbial products (e.g., LPS) to enter the circulation. 2. These molecules are “sensed” by pattern recognition receptor enriched in immune cells, such as neutrophils (not shown) and macrophages (shown). An increase in macrophage activation leads to production and release of cytokines (e.g., IL-1β). 3. Distinct pro-inflammatory cytokines (e.g., IL-1β, IFN-γ, TNF-α, etc.) promote transcriptionally active NF-κB and STAT1 proteins. 4. The NF-κB and STAT1 pathways regulate chemokine production in beta-cells; release of β-cell derived chemokines influences innate and adaptive immune responses.
B. 1. When consumption of free fatty acids (FFAs) exceeds storage capacity in adipose tissue, these lipids begin to be stored in lean tissues (e.g. liver, muscle, and pancreas). 2. FFAs cell surface receptors, altering intracellular signaling pathways. 3. Incomplete fatty acid oxidation may also activate signaling pathways, such as NF-kB, that are linked with transcriptional changes. 4. Chemokine production is elevated in the obese, insulin resistant state, which would impact both tissue resident and infiltrating leukocytes.
