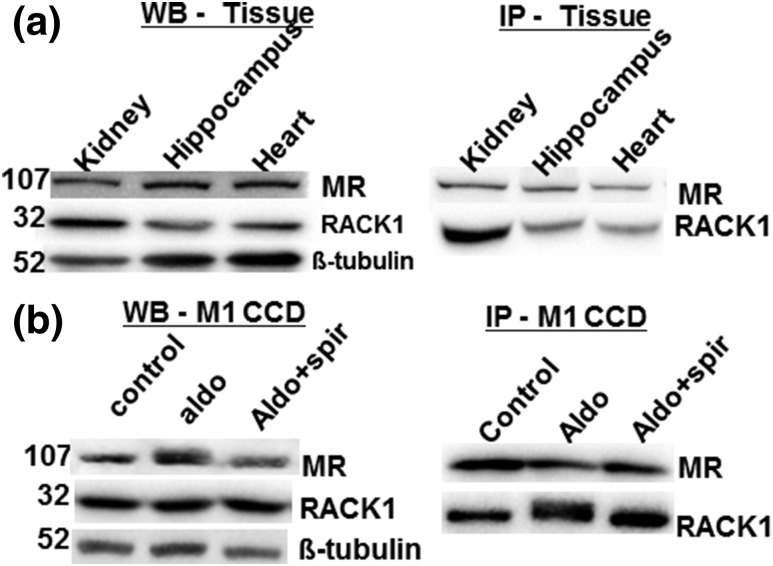
Figure 1.
RACK1 interacts with MR in rat tissue and mouse collecting duct cells. (a) Coimmunoprecipitation of RACK1 and MR in the rat kidney, hippocampus, and heart samples. Tissue lysates (30 µg) were separated by western blot (WB), and MR (1D5), RACK1, and β-tubulin as internal control (left panel) were immunodetected. For immunoprecipitation (IP), whole-cell extracts (800 µg) were incubated with anti-MR antibody and immunoprecipitates were subsequently analyzed by western blot with anti-RACK1 and anti-MR antibodies (right panel). (b) The M1-rMR TAT3-Gluc cells treated with aldosterone (10 nM) and aldosterone (10 nM) with spironolactone (1 µM) for >3 hours and then total protein (30 µg) were separated, and MR and RACK1 proteins were detected by western blot (left panel). Whole-cell lysates were immunoprecipitated with an anti-MR antibody and detected with an anti-RACK1 antibody (right panel). Aldo, aldosterone; Aldo+spiro, aldosterone with spironolactone.