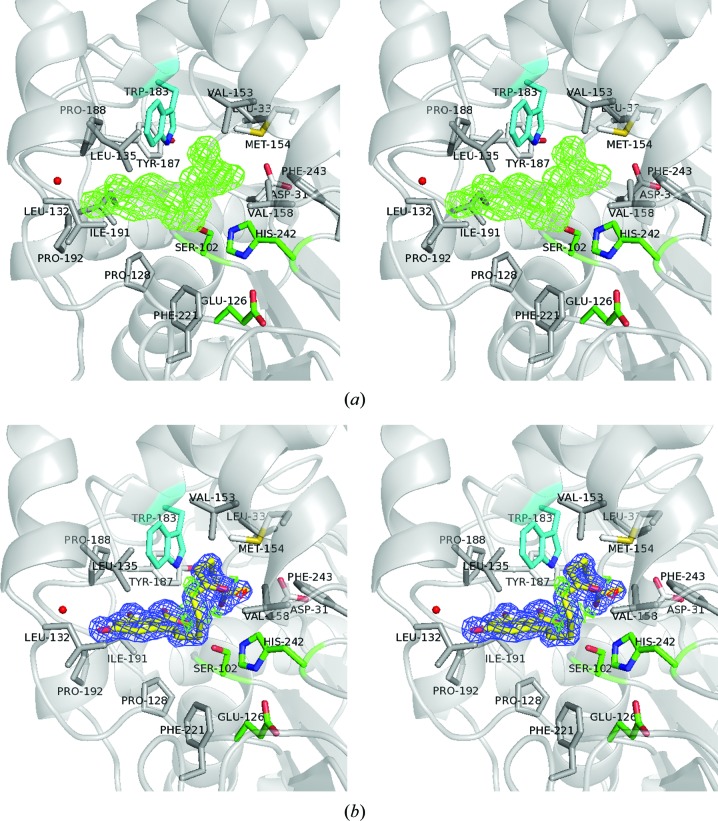
Figure 2.
The ZGR molecule in the substrate-binding pocket. (a) Wall-eyed stereo presentation of the initial difference map. The F o − F c difference density OMIT map contoured at 3.0σ (green) clearly shows the skeleton of ZGR. (b) Wall-eyed stereo presentation of the ZGR structure. The 2F o − F c electron-density map contoured at 1.5σ is shown in blue. The F o − F c map contoured at ±3.0σ is shown in green and red, respectively. The catalytic triad Ser102–His242–Glu126 is shown in green. The environment surrounding ZGR shows the hydrophobic interactions with amino acids (grey sticks) and the hydrophilic interactions of ZGR with Ser102, Trp183 (in cyan) and one water molecule (red sphere).