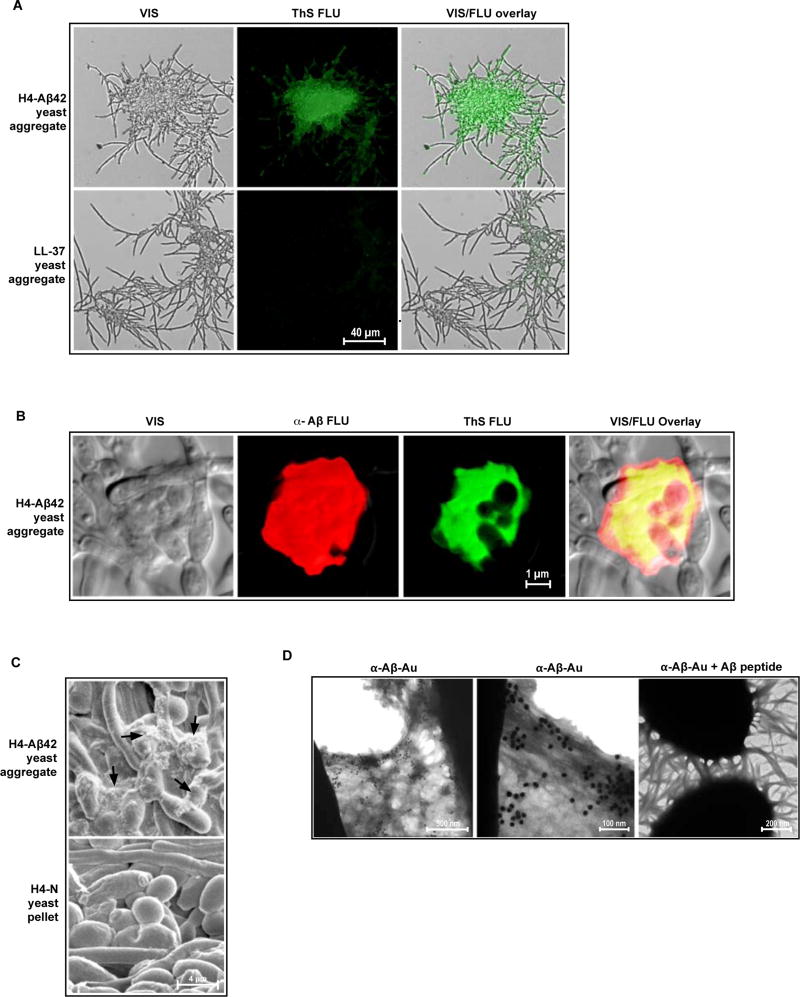
Fig. 5. Candida cells are entrapped by β-amyloid in H4-Aβ42 culture media.
Following overnight incubation with H4-Aβ42 media, yeast (C. albicans) aggregates were harvested and probed for β-amyloid markers. (A and B) Visible yeast aggregates (VIS), yeast aggregates stained with green-fluorescence Thioflavin S (Thioflavin S FLU), yeast aggregates stained with red-fluorescence anti-Aβ (α-Aβ FLU); superpositioned images (VIS/FLU overlay). Yeast aggregates generated with the control synthetic LL-37 peptide (A) are negative for Thioflavin S enhanced fluorescence. (B) Yellow denotes co-localization of anti-Aβ and Thioflavin S signals. Co-localization of these signals is the hallmark of β-amyloid. (C) SEM analysis revealed fibrous material in H4-Aβ42 yeast aggregates that is absent from control C. albicans pellets prepared by centrifugation in H4-N media. (D) H4-Aβ42 yeast aggregates incubated with immunogold nanoparticles coated with anti-Aβ antibodies (α-Aβ-Au) and analyzed by TEM. First and second panels show labeling of fibrous material by α-Aβ-Au. Third panel shows inhibition of α-Aβ-Au nanoparticle binding by soluble synthetic Aβ peptide (α-Aβ-Au + Aβ peptide), consistent with specific labeling of β-amyloid. Micrographs are representative of data from two or more replicate experiments and multiple discrete image fields (table S1A).