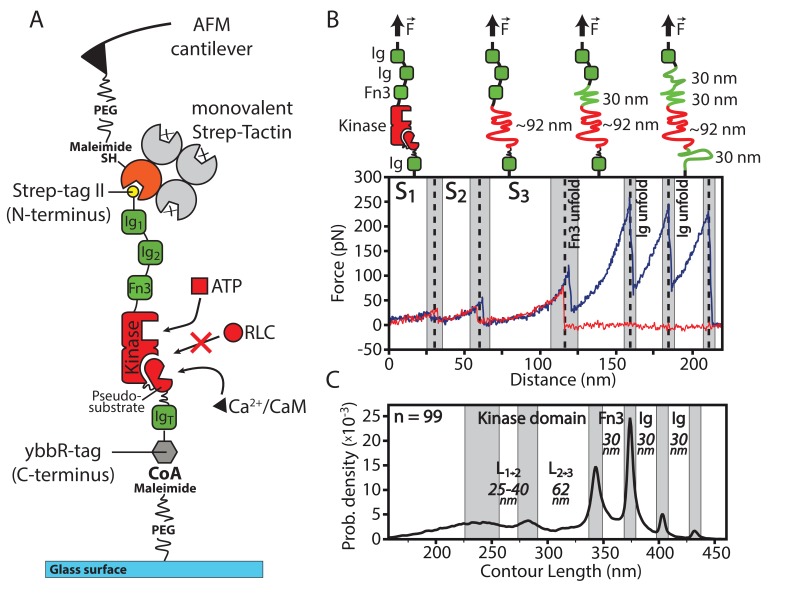
Figure 1. Overview of the experimental configuration for applying controlled mechanical stress to smMLCK.
(A) Schematic illustration of the investigated smMLCK construct. It consists of the kinase domain surrounded by several Ig-like domains (Ig) and a fibronectin-like domain (Fn3). Possible substrate interactions are indicated (ATP, Ca2+/CaM and RLC). RLC interaction is prevented by the auto-inhibitory pseudosubstrate sequence that is released upon Ca2+/CaM binding. For covalent attachment onto the surface, the construct harbors a C-terminal ybbR-tag. (B) Representative force-distance curves (red, blue) depicting the characteristic transitions of the kinase through different conformational states (S, S, S) and subsequent unfolding of the adjacent Fn3 and Ig-like domains. Whereas most force-distance curves rupture before or after Fn3 unfolding (as shown in red) due to comparable rupture forces of Fn3 and the employed handle system, the blue curve illustrates a descriptive example with additional unfolding of Fn3 and Ig-like domains depicting the further force-distance pattern given by the construct. Structural interpretation and assignment of the detected force-distance pattern is schematically depicted above the curve. (C) Contour length transformation of 99 unfolding events with respective contour length increments. L and L are released at the transition of the kinase domain from conformational state S to S and S to S respectively. The contour lengths of Fn3 and Ig-like domains are additionally depicted.
DOI: http://dx.doi.org/10.7554/eLife.26473.002