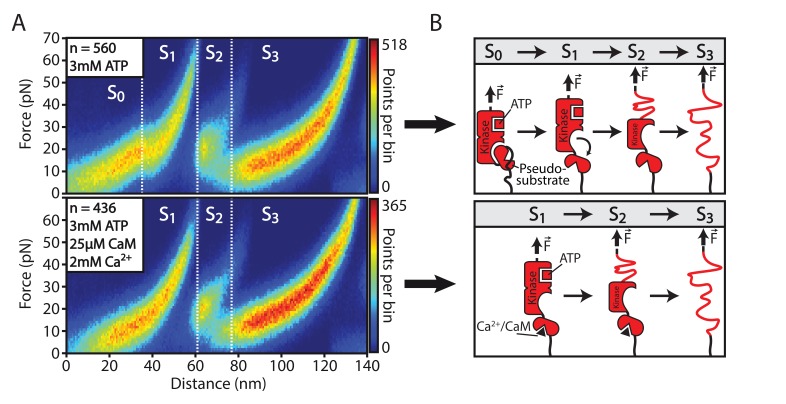
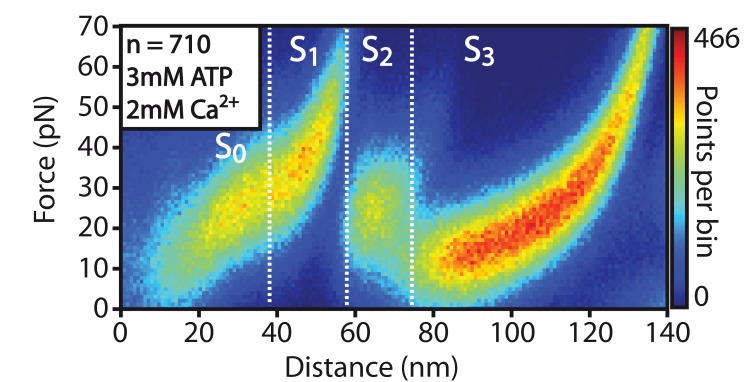
Figure 3. Structural effects of Ca2+/CaM binding on smMLCK’s characteristic sequence of conformational states.
(A) Attenuated S→S transition in the characteristic force-distance pattern of the smMLCK construct due to conformational changes upon Ca2+/CaM binding. This effect is emphasized by a heatmap comparison of several hundred overlaid force-distance curves. Both data sets were collected within one measurement. (B) Structural model interpretation. The S→S transition is assigned to a force-induced rearrangement in the kinase domain that correlates with the conformational changes induced by Ca2+/CaM binding – the release of the inhibitory pseudosubstrate.