Summary
Introduction
Complex regional pain syndrome (CRPS) is a major complication after trauma, surgery, and/or immobilization of an extremity. The disease often starts with clinical signs of local inflammation and develops into a prolonged phase that is characterized by trophic changes and local osteoporosis and sometimes results in functional impairment of the affected limb. While the pathophysiology of CRPS remains poorly understood, increased local bone resorption plays an undisputed pivotal role. The aim of this retrospective clinical study was to assess the bone microstructure in patients with CRPS.
Methods
Patients with CRPS type I of the upper limb whose affected and unaffected distal radii were analyzed by high-resolution peripheral quantitative computed tomography (HR-pQCT) were identified retrospectively. The osteology laboratory data and dual-energy X-ray absorptiometry (DXA) images of the left femoral neck and lumbar spine, which were obtained on the same day as HR-pQCT, were extracted from the medical records.
Results
Five patients were identified. The CRPS-affected upper limbs had significantly lower trabecular numbers and higher trabecular thicknesses than the unaffected upper limbs. However, the trabecular bone volume to total bone volume and cortical thickness values of the affected and unaffected sides were similar. Trabecular thickness tended to increase with time since disease diagnosis.
Discussion
CRPS associated with significant alterations in the bone microstructure of the affected upper limb that may amplify as the duration of disease increases.
Keywords: complex regional pain syndrome, CRPS, bone microstructure, HR-pQCT
Introduction
Complex regional pain syndrome (CRPS), also named Sudeck’s atrophy or reflex sympathetic dystrophy, is a chronic pain condition that most often affects a limb. It is characterized by prolonged or excessive pain and mild to dramatic changes in skin color, temperature, and/or swelling in the affected area. It is thought to be caused by damage to, or malfunction of, the peripheral or central nervous systems and is a major complication of trauma or surgery to an extremity. Its incidence ranges from 5.5 cases per 100,000 persons per year in the United States of America (1) to 26.2 per 100,000 persons per year in the Netherlands (2). However, these estimates be may underestimates because the clinical signs of CRPS often fluctuate, which can hamper its diagnosis. The disease affects women three times more frequently than men.
There are two forms of CRPS. Type I is the more common form and is a severely disabling pain syndrome that is characterized by allodynia, hyperalgesia, and interstitial edema. Later, it can manifest as trophic changes such as osteoporosis. Ultimately, it can impair the function of the affected limb. CRPS type II is the same syndrome except that, unlike type I, it associates with obvious peripheral nerve injury. The pathophysiology of CRPS is not fully understood, but fractures account for up to 50% of the type I cases (3). The disease can be divided into an early phase that is characterized by clinical signs of inflammation (dolor, tumor, rubor, calor, and functio laesa) and a later phase that is characterized by the trophic changes described above. The disease is diagnosed on the basis of clinical signs only as laboratory data or radiological imaging are only helpful for excluding other diagnoses (4). The updated Budapest Criteria are widely accepted for the diagnosis of CRPS (5).
The pathophysiology in CRPS is discussed in detail elsewhere but, briefly, it involves both the sympathetic nervous system (6, 7) and local inflammatory processes such as the production of nerve growth factor (NGF), which shapes the liberation of neuropeptides (8). Bone resorption also plays a pivotal and well-accepted role in the pathophysiology of CRPS. Indeed, several recent randomized controlled trials showed that bisphosphonate treatment improves pain and other clinical features of CRPS (9–11). The primary mechanism by which bisphosphonates improve CRPS is osteoclast inhibition. They may also act by reducing the local production of lactic acid, inhibiting macrophage function, and reducing the production of NGF and other cytokines (12).
Despite these pathophysiological findings, the effects of CRPS on histological bone morphology remain poorly understood (13). One way to determine these effects would be to use high-resolution peripheral quantitative computed tomography (HR-pQCT), as this technology permits the in vivo assessment of trabecular and cortical architecture at the distal radius and distal tibia (14). This technology is important because the two compartments may be affected in different ways by different diseases.
In this retrospective clinical study, we aimed to improve our understanding of the bone remodeling processes in CRPS by examining the HR-pQCT findings of 5 patients with CRPS who had undergone HR-pQCT of both the affected and unaffected upper limbs. The aim was to determine the bone microstructure of the affected limb. In particular, we asked whether either or both the cortical and trabecular compartments are primarily affected in CRPS.
Methods
Patients
This retrospective study was based on all patients with CRPS of the upper limb who underwent HR-pQCT of both the affected and unaffected distal radii between 2013 and 2015 in the Department of Osteology and Biomechanics, at the University Medical Centre Hamburg-Eppendorf, Germany. All patients were clinically diagnosed with CRPS on the basis of the updated Budapest Criteria (5).
HR-pQCT
The affected and unaffected distal radii were assessed by HR-pQCT (XtremeCT, SCANCO Medical, Brüttisellen, Switzerland) using the default in vivo settings, namely, 60 kVp, 1,000 μA, 100 ms integration time, and resolution of 82 μm. These settings generate 3-dimensional microstructural data of the cortical and trabecular compartments (Figure 1). Before starting the measurement, the forearm was placed in an appropriate cast and fixed with two Velcro straps. To determine the measurement area, a scout view with a reference line was set. For the correct and standardized position of the reference line, the endplate of the radius was used. The region of interest started 9.5 mm proximal to the reference line with following 110 slices. The manufacturer’s standard protocol was used to analyze the bone microstructure, including trabecular bone volume to total bone volume (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), cortical thickness (C.Th) and cortical bone mineral density (C.BMD). The same technician scanned and analyzed the HR-pQCT images using the standard manufacturer’s method as described previously (15). Quality control was performed using a daily calibration phantom that was provided by the manufacturer.
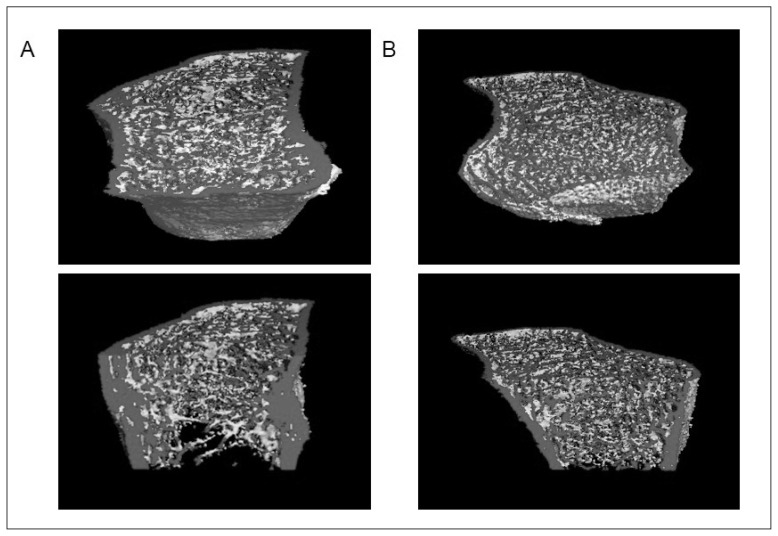
Figure 1.
High-resolution quantitative computed tomography images of the distal radii of a patient with chronic regional pain syndrome (CRPS). (A) The CRPS-affected distal radius. (B) The unaffected distal radius. Note the decreased trabecular number and increased trabecular thickness in the CRPS-affected radius compared to that in the unaffected radius.
Laboratory and bone mineral density (BMD) values
The dual-energy X-ray absorptiometry images of the left femoral neck and lumbar spine, and the calcium, phosphorus, parathyroid hormone, creatinine, and vitamin D levels of the patients at the time of the HR-pQCT measurements, were extracted from the records.
Statistics
Continuous and categorical variables were expressed as mean ± standard deviation (range) and n, respectively. The affected and unaffected radii were compared in terms of mean BV/TV, Tb.N, Tb.Th, C.Th and C.BMD using paired Student’s t-test. P values of 0.05 were considered to indicate statistical significance. The IBM SPSS statistics 22 program was used for statistical analyses.
Results
In the study period, 5 patients with CRPS of the distal radius underwent HR-pQCT of both the affected and unaffected distal radii. Four were women and 1 was male. The average age was 51.6 years. Three patients had developed CRPS after distal radius fracture. The remaining 2 patients had developed CRPS after operative treatment of rhizarthrosis by trapezectomy and after wound debridement for dog bite infection, respectively. The average disease duration until HR-pQCT was 19.7 months (Table 1).
Table 1.
Demographic and laboratory characteristics of patients with chronic regional pain syndrome at the time performing high-resolution quantitative computed tomography.
| Patients | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Gender (m/f) | f | f | f | m | f | |
| Age (years) | 66 | 23 | 58 | 36 | 66 | |
| Weight (kg) | 54 | 60 | 81 | 72 | 54 | |
| Height (cm) | 170 | 175.5 | 168 | 182 | 157 | |
| Body mass index (kg/m2) | 18.7 | 19.6 | 28.7 | 21.7 | 21.9 | |
| Side affected by CRPS (left/right) | left | left | left | right | left | |
| Dual X-ray absorptiometry | ||||||
| T-score lumbar spine | 1.1 | −0.3 | −0.7 | −3.2 | −4.1 | |
| T-score score left femur | −1.4 | 1.1 | 0.2 | −1.5 | −2.7 | |
| Laboratory data | Reference | |||||
| Calcium (mmol/l) | 2.08 | 2.27 | 2.30 | 2.39 | 2.34 | 2.13–2.63 |
| Phosphate (mmol/l) | 0.98 | 0.99 | 0.90 | 0.89 | 0.87 | 0.77–1.50 |
| Parathyroid hormone (ng/l) | 88.06 | 40.46 | 25.06 | 22.30 | 30.66 | 22.0–109.0 |
| Creatinine (mg/dl) | 0.89 | 0.83 | 1.0 | 0.80 | 0.5 | 0.6–1.2 |
| 25-OH-D3 (ug/l) | 27.49 | 27.30 | 24.70 | 36.60 | 35.20 | >30 |
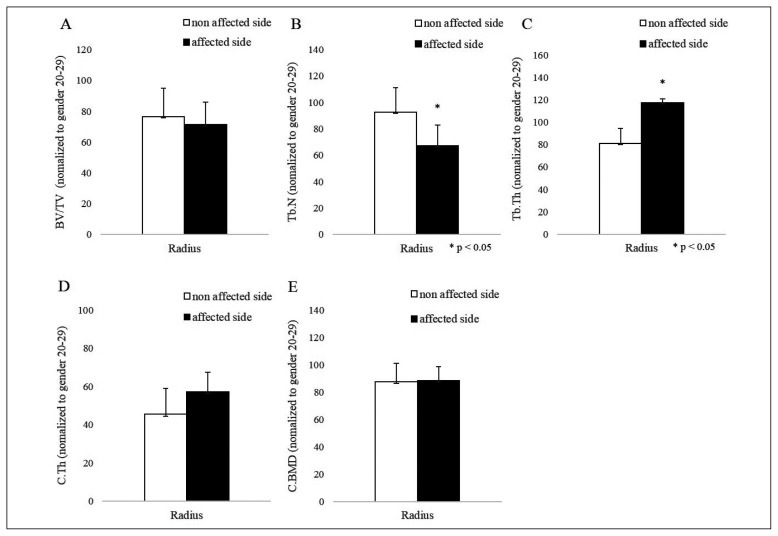
The affected and unaffected radii did not differ significantly in terms of trabecular bone volume relative to total bone volume (71.28 vs 76.69%), cortical thickness (57.35 vs 45.53%) or cortical BMD (88.72 vs 87.62%), but the CRPS-affected limb had significantly lower trabecular numbers (67.32 vs 92.73%, p=0.049) and higher trabecular thickness (117.33 vs 81.04%, p=0.040) (Figures 1, 2).
Figure 2.
Trabecular and cortical bone microstructure of the patients with chronic regional pain syndrome (CRPS). (A) Trabecular bone volume relative to total bone volume (BV/TV), (B) trabecular number (Tb.N), (C) trabecular thickness (Tb.Th), (D) cortical thickness (C.Th), and (E) cortical BMD (C.BMD) at the CRPS-affected (black bars) and unaffected (white bars) distal radii. All values are means ± SEM (*p<0.05, as determined by paired Student’s t-test).
Analysis of the relationship between trabecular bone microstructure and duration of disease before HR-pQCT showed that, relative to the unaffected radius, the Tb.Th of the CRPS-affected radius tended to increase over time since disease onset while its Tb.N tended to decrease at the beginning (Table 2). For example, the patients who were diagnosed with CRPS more than 10 months before HR-pQCT had 40–70% greater Tb.Th on the affected side compared to the unaffected side, whereas patients diagnosed earlier exhibited 0–27% increases in Tb.Th in the affected radius.
Table 2.
Differences in bone microstructure in percent between the CRPS affected and unaffected sides.
| Patient | Disease duration until HR-pQCT (month) | BV/TV | Tb.Th | Tb.N | C.Th | C.BMD |
|---|---|---|---|---|---|---|
| 1 | 1.5 | −4.76% | −1.02% | −3.13% | −19.82% | −22.08% |
| 2 | 6 | 15.56% | −17.55% | 27.13% | 12.89% | 1.16% |
| 3 | 12 | −28.57% | −55.38% | 70.31% | −21.70% | −16.12% |
| 4 | 19 | −7.69% | −33.64% | 40.26% | 52.83% | 23.67% |
| 5 | 60 | −1.59% | −19.48% | 46.88% | 34.90% | 18.88% |
The laboratory data at the time HR-pQCT was performed showed that the serum levels of calcium, vitamin D, phosphorus, creatinine, and parathyroid hormone were within the normal range (Table 1). Two patients had osteoporosis, as indicated by the BMD (as measured by dual-energy X-ray absorptiometry): in both patients, the T-Score at the lumbar spine was <−2.5. A third patient had osteopenia. The remaining 2 patients had normal BMD (Table 1). Relationships between the bone microstructure changes at the time of HR-pQCT and the laboratory data or the BMD were not found.
Discussion
This retrospective clinical study showed for the first time how the bone microstructure in the affected distal radius of patients with CRPS changes relative to the bone microstructure in the unaffected distal radius. The affected radii exhibited significantly lower trabecular number and significantly greater trabecular thickness than the unaffected radii. There was a tendency for both of these changes to amplify over time since disease onset. In particular, patients who had CRPS for at least 10 months had markedly greater trabecular thickness on the affected side relative to the unaffected side, whereas this change was much less prominent in the patients who had been diagnosed more recently. These findings probably reflect trabecular hyperthrophy that follows the reduction of trabecular number and aims to preserve the trabecular bone volume relative to total bone volume. Indeed, the affected and unaffected radii of the patients had similar trabecular bone volume to total bone volume.
The reason for the increased bone resorption in CRPS remains unclear. It may reflect perturbation of the centrally controlled bone remodeling processes, which are mediated by a neuroendocrine mechanism (6) and the sympathetic nervous system. This notion is supported by the well-established fact that osteoblasts and osteoclasts are equipped with adrenergic and peptidergic receptors. This suggests that these cells are innervated by sympathetic and sensory nerves and their activity could be modulated by changes in the activity of these nerves (7). This possibility is supported by the observation that the beta-2 adrenergic receptors in osteoblasts inhibit their activity; moreover, adrenergic nerves can activate the RANKL receptor in osteoclasts and trigger RANKL-mediated osteoclastogenesis that manifests as bone resorption (7). Another possible mechanism that drives the increased bone resorption in CRPS is neurogenic inflammation, which is mainly mediated by NGF. This neuropeptide is produced by leukocytes and activated mast cells, binds to specific receptors on sensitive afferent fibers, and shapes the release of neuropeptidases, which in turn alter the levels of substance P and calcitonin gene-related peptide (8). This in turn may contribute to the clinical presentation of CRPS, since these neuropeptidases induce local vasodilatation, increase capillary permeability, and promote interstitial edema (16). These effects in turn generate local hypoxia with acidosis. This cascade of events could further promote the local inflammatory process by causing occlusion of the local vessel lumen, which in turn induces the formation of arteriovenous shunts that aggravate the hypoxic stress and capillary damage (17).
Most of the studies on the histopathology of CRPS only analyzed the superficial tissue (skin and subcutaneous tissues) of the affected limb: studies on the CRPS-related changes in joints and bone have not yet been published. In addition, while there are some animal models for CRPS that have been used to assess the histopathological effect of trophic changes, only a few studies using these models have been published (18). An excellent technique for assessing bone histopathology is HR-pQCT, which provides images of the trabecular and cortical bone. This digital assessment of the bone microstructure in vivo improves the evaluation of bone diseases and was therefore used for many studies in the last few years. Previous studies show that HR-pQCT provides high-resolution 3-dimensional reconstructed in vivo images of cortical and trabecular bone while exposing the patients to only low levels of radiation. It thus can be used to detect age- and disease-related changes in trabecular microarchitecture (19). Krause et al. also showed in 2014 that HR-pQCT provides acceptable in vivo accuracy for BV/TV in patients with osteoporosis and bisphosphonate treatment compared to the gold standard (microtomography) (20). MacNeil et al. (21) also found that HR-pQCT accurately reflects microtomography of biopsies taken from the same regions of cadaveric radii. To our knowledge, HR-pQCT had never been used to assess the CRPS-associated changes in bone microstructure previously.
As mentioned above, the increased local bone resorption in CRPS may reflect local inflammatory processes. However, several lines of evidence suggest that these processes differ from the inflammatory processes that drive the loss of bone, for example in rheumatoid arthritis (RA). First, when Zhu et al. (22) subjected the distal radius of patients with RA to HR-pQCT, they found this disease associated with a deleterious effect on the cortical bone rather than the trabecular bone. Second, Fouque-Aubert et al. (23) showed with HR-pQCT in 2010 that the metacarpal phalange heads of patients with RA exhibited decreased trabecular thickness (the effect of RA on cortical measurements was not assessed because of the thin cortical bone at the metacarpal phalange heads). By contrast, in our study, we found that the CRPS-associated changes occurred in the trabecular compartment, not the cortical compartment and the changes were associated with increased trabecular thickness, not decreased thickness. To our best knowledge the influence of dominant side on microstructure bone values measured by HR-pQCT have not yet been performed. In our study the cortical bone mineral density was similar in both forearms and thus may reflect similar cortical bone architecture in dominant and non-dominant side. Previous studies demonstrated similar bone density in dominant and non-dominant forearms (24) and reasoned that the consistency in scan acquisition techniques and scan analyses is of greater importance than the selection of an extremity based on hand dominance in DXA studies (25), whereas others found higher BMD in the dominant metacarpal phalange of patients (26). However, further HR-pQCT studies are needed to analyse the dominant side effect on bone architecture.
This study has a number of limitations. First, the sample size was small and the retrospective study design prohibited follow-up of the patients. Second, in three of the five patients, a fracture was probably the reason for the CRPS. This could suggest that the bone remodeling pattern detected by HR-pQCT reflects the fracture rather than CRPS. However, this possibility is not supported by the data of the two non-fracture patients: both exhibited similar relative reductions in trabecular number and increases in trabecular thickness in the CRPS-affected limb to the 3 fracture patients.
In summary, we demonstrated for the first time, using HR-pQCT, the in vivo changes in the bone microstructure of the upper limb in patients with CRPS. This is not only helpful for improving our understanding of the CRPS-related changes in bone microstructure for future studies, but will also be useful for monitoring the effect of treatments on bone, especially bisphosphonate treatment, which is being increasingly used to treat CRPS.
Footnotes
Conflict of interest
All Authors declare that they have no conflict of interest.
References
- 1.Sandroni P, Benrud-Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain. 2003;103:199–207. doi: 10.1016/s0304-3959(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 2.De Mos M, de Bruijn AGJ, Huygen FJPM, et al. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129:12–20. doi: 10.1016/j.pain.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Giusti A, Bianchi G. Treatment of complex regional pain syndrome type I with bisphosphonates. RMD Open. 2015;1:e000056. doi: 10.1136/rm-dopen-2015-000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatti D, Rossini M, Adami S. Management of patients with complex regional pain syndrome type I. Osteoporos Int. 2016 doi: 10.1007/s00198-016-3531-9. [DOI] [PubMed] [Google Scholar]
- 5.Harden RN, Bruehl S, Perez RS, et al. Validation of proposed diagnostic criteria (the “Budapest Criteria”) for complex regional pain syndrome. Pain. 2010;150:268–274. doi: 10.1016/j.pain.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amling M, Takeda S, Karsenty G. A neuro (endo)crine regulation of bone remodeling. Bioessays. 2000 Nov;22(11):970–975. doi: 10.1002/1521-1878(200011)22:11<970::AID-BIES3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Elefteriou F, Ahn JD, Takeda S, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 8.Schinkel C, Gaertner A, Zaspel J, et al. Inflammatory mediators are altered in the acute phase of posttraumatic complex regional pain syndrome. Clin J Pain. 2006;22:235–239. doi: 10.1097/01.ajp.0000169669.70523.f0. [DOI] [PubMed] [Google Scholar]
- 9.Varenna M. Bisphosphonates beyond their anti-osteoclastic properties. Rheumatology (Oxford) 2014;53:965–967. doi: 10.1093/rheumatology/ket370. [DOI] [PubMed] [Google Scholar]
- 10.Varenna M, Adami S, Rossini M, et al. Treatment of complex regional pain syndrome type I with neridronate: a randomized, double-blind, placebo-controlled study. Rheumatology (Oxford) 2013;52:534–542. doi: 10.1093/rheumatology/kes312. [DOI] [PubMed] [Google Scholar]
- 11.Robinson JN, Sandom J, Chapman PT. Efficacy of pamidronate in complex regional pain syndrome type I. Pain Med. 2004;5:276–80. doi: 10.1111/j.1526-4637.2004.04038. [DOI] [PubMed] [Google Scholar]
- 12.Giusti A, Bianchi G. Treatment of complex regional pain syndrome type I with bisphosphonates. RMD Open. 2015;1:e000056. doi: 10.1136/rm-dopen-2015-000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMahon SB, Jones NG. Plasticity of pain signaling: role of neurotrophic factors exemplified by acid-induced pain. J Neurobiol. 2004;61:72–87. doi: 10.1002/neu.20093. [DOI] [PubMed] [Google Scholar]
- 14.Laib A, Hauselmann HJ, Ruegsegger P. In vivo high resolution 3D-QCT of the human forearm. Technol Health Care. 1998;6:329–337. [PubMed] [Google Scholar]
- 15.Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010;47(3):519–528. doi: 10.1016/j.bone.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harden RN. Cytokine imbalance/activity as a unifying hypothesis for the pathogenesis and pathophysiology of Complex Regional Pain Syndrome? Pain. 2011;152:247–248. doi: 10.1016/j.pain.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Birklein F, Schmelz M. Neuropeptides, neurogenic inflammation and complex regional pain syndrome (CRPS) Neurosci Lett. 2008;437:199–202. doi: 10.1016/j.neulet.2008.03.081. [DOI] [PubMed] [Google Scholar]
- 18.Sabsovich I, Guo TZ, Wei T, et al. TNF signaling contributes to the development of nociceptive sensitization in a tibia fracture model of complex regional pain syndrome type I. Pain. 2008;137:507–519. doi: 10.1016/j.pain.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In Vivo Assessment of Trabecular Bone Microarchitecture by High-Resolution Peripheral Quantitative Computed Tomography. J Clin Endocrinol Metab. 2005 Dec;90(12):6508–6515. doi: 10.1210/jc.2005-1258. Epub 2005 Sep 27. [DOI] [PubMed] [Google Scholar]
- 20.Krause M, Museyko O, Breer S, Wulff B, Duckstein C, Vettorazzi E, Glueer C, Püschel K, Engelke K, Amling M. Accuracy of trabecular structure by HR-pQCTcompared to gold standard μCT in the radius and tibia of patients with osteoporosis and long-term bisphosphonate therapy. Osteoporos Int. 2014;25:1595–1606. doi: 10.1007/s00198-014-2650-4. [DOI] [PubMed] [Google Scholar]
- 21.MacNeil JA, Boyd SK. Accuracy of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys. 2007;29:1096–1105. doi: 10.1016/j.medengphy.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Tracy Y, Griffith James F, Qin Ling, Hung Vivian WY, Fong Tsz-Ning, Au Sze-Ki, Tang Xiao Lin, Kwok Anthony W, Leung Ping-Chung, Li Edmund K, Tam Lai-Shan. Structure and strength of the distal radius in female patients with rheumatoid arthritis: a case-control study. J Bone Miner Res. 2013 Apr;28(4):794–806. doi: 10.1002/jbmr.1793. [DOI] [PubMed] [Google Scholar]
- 23.Fouque-Aubert A, Boutroy S, Marotte H, Vilayphiou N, Bacchetta J, Miossec P, Delmas PD, Chapurlat RD. Assessment of hand bone loss in rheumatoid arthritis by high-resolution peripheral quantitative CT. Ann Rheum Dis. 2010 Sep;69(9):1671–1676. doi: 10.1136/ard.2009.114512. Epub 2010 Jun 4. [DOI] [PubMed] [Google Scholar]
- 24.Min JY, et al. Side differences in the bone density of the distal radius and calcaneus in Koreans aged 4–86 years. J Clin Densitom. 2007;10(2):184–188. doi: 10.1016/j.jocd.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Walters J, et al. Effect of hand dominance on bone mass measurement in sedentary individuals. J Clin Densitom. 1998;1(4):359–367. doi: 10.1385/jcd:1:4:359. [DOI] [PubMed] [Google Scholar]
- 26.Lekamwasam S, et al. Comparison of phalangeal bone mineral content and density between the dominant and non-dominant sides. Ceylon Med J. 2005;50(4):149–151. doi: 10.4038/cmj.v50i4.1404. [DOI] [PubMed] [Google Scholar]