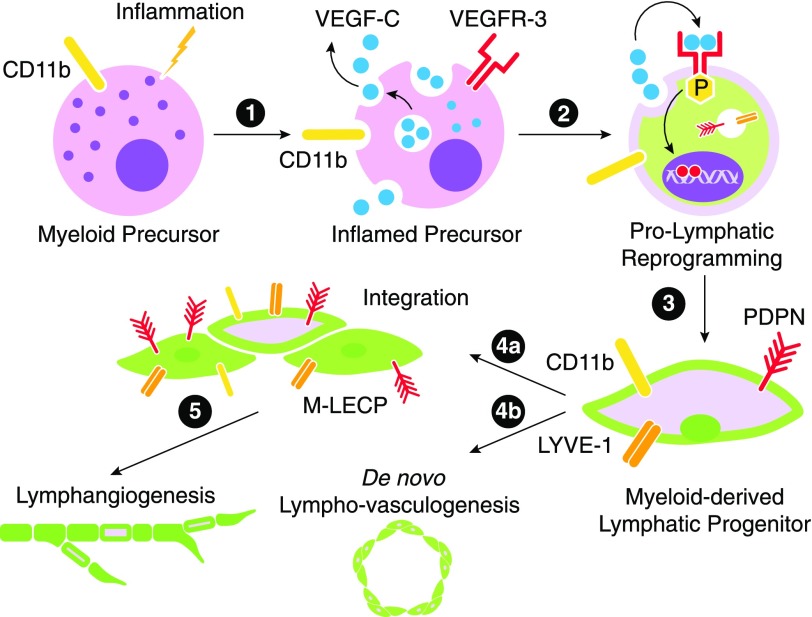
Figure 1. A model for inflammation-induced reprogramming of immature myeloid cells into M-LECP.
Inflammatory stimuli reach the BM, where they activate the NF-κB pathway in HSCs/hematopoietic progenitors and immature myeloid cells. NF-κB activation could be mediated by TLR4 or endothelial receptors if they are already expressed in myeloid cells. (1) Activation of the NF-κB pathway leads to simultaneous up-regulation of VEGFR-3 and its ligand VEGF-C. (2) Coexpression of VEGF-C and VEGFR-3 creates an autocrine loop leading to up-regulation of lymphatic-specific genes. Prolymphatic reprogramming generates M-LECPs, which coexpress monocytic (e.g., CD11b) and lymphatic-specific (e.g., Lyve-1 and PDPN) markers that are normally segregated to distinct cell lineages. (3) Under chronic inflammatory conditions or cancer, BM-generated M-LECPs are mobilized to peripheral blood. (4) Circulating M-LECPs are recruited to inflamed sites and tumors and either integrate into pre-existing lymphatic vessels (4a) or initiate de novo formation of new lymphatic vasculature (i.e., lympho-vasculogenesis; 4b). (5) M-LECP integration might be necessary to induce sprouting of existing vessels that otherwise might be resistant to undergo remodeling.