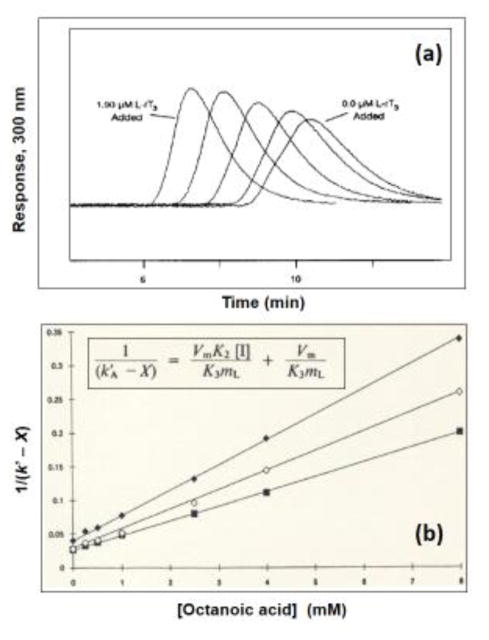
Figure 3.
Examples of competition studies carried out by zonal elution. The chromatograms in (a) were obtained on an HPLC column containing immobilized HSA by using R-warfarin as the injected solute and mobile phases that contained L-reverse triiodothyronine (L-rT3) at concentrations (from right-to-left) of 0.0, 0.24, 0.49, 0.97 or 1.90 μM. The plot in (b) shows the effects of adding octanoic acid to the mobile phase on the observed retention for (◆) R-warfarin, (◇) S-warfarin, and (■) phenylbutazone on an HPLC column containing immobilized HSA. The terms used within the equation in (b) are defined as follows: k′A is the retention factor for the injected solute, X is the retention for this solute due to sites other than those involved in the competitive binding with the mobile phase additive (i.e., octanoic acid), [I] is the mobile phase concentration of the additive, Vm is the void volume, mL is the moles of sites that are involved in the competition process, and K3 or K2 are the association equilibrium constants for the injected solute or mobile phase additive at the site of competition. Adapted with permission from Refs. [51,54].