Figure 1.
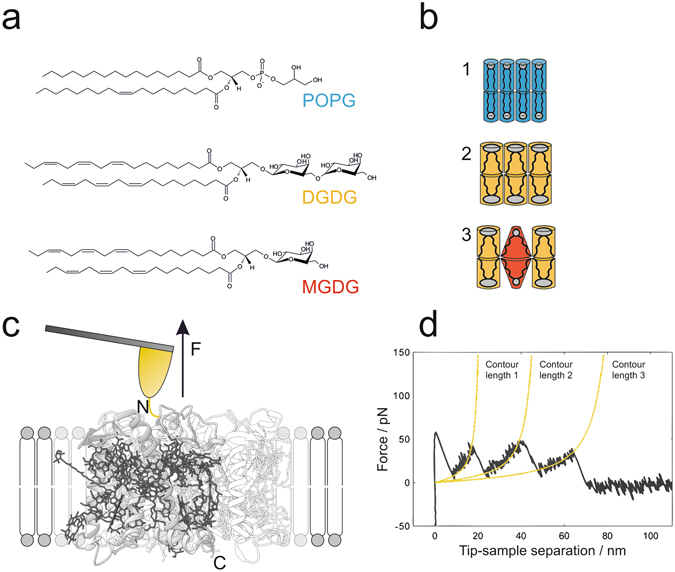
Mechanical unfolding of LHCII in membranes assembled from thylakoid lipids. (a) Chemical structures of model phospholipid POPG and thylakoid glycolipids DGDG and MGDG. (b) Lipid geometries corresponding to (a) and lipid compositions of the membranes applied for unfolding experiments (1–3). The degree of saturation in the alkyl chain region and the size of the head group determine the shape of lipids. Hence, POPG (blue) and DGDG (orange) adopt a cylindrical shape, forming lamellar membranes (1,2). Due to its conical shape the non-bilayer lipid MGDG (red) causes alterations in the lateral pressure profile upon incorporation into lamellar membranes23, 24; here: lipid ratio of DGDG / MGDG = 2:1 (3). (c) Schematic representation of SMFS with LHCII embedded in lipid bilayers with compositions according to (b). Site-directed unfolding of LHCII is achieved by covalently attaching a gold-coated AFM tip to a cysteine motif (yellow) at the amino-terminus N of the polypeptide. Retraction of the tip applies a pulling force F to LHCII monomers, which get extracted from the membrane out of their trimeric assembly (transparent presentation), thereby inducing stepwise unfolding of the protein; gray: polypeptide, dark grey: pigments (obtained with Chimera and PDB ID code 2BHW). (d) Exemplary force-distance curve recorded during the unfolding process described in (c). Each force peak, representing an unfolding event of LHCII, is fitted with the WLC model (yellow) which provides information about the positions of the stabilized domains (contour lengths) and the forces needed to overcome these barriers along the LHCII polypeptide.
