Figure 2.
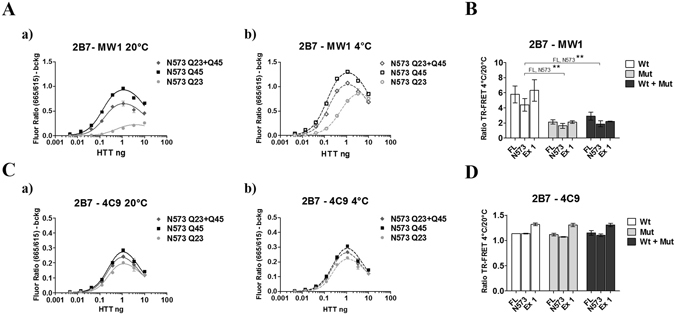
The 2B7/MW1 conformational immunoassay preferentially detects mHTT conformation in cocktails of isolated HTT proteins with wild type or mutant polyQ. (A) The 2B7/MW1 TR-FRET assay performed on wild type HTT, mHTT or a cocktail (of equivalent final protein concentration) of the two proteins at 20 °C (a) or measured after shifting the same samples to 4 °C (b). For simplicity, only results for a representative experiment performed on the N573 HTT protein fragments are shown. (B) Summary of data (ratio TR-FRET signals at 4 °C/20 °C) obtained on individual isolated HTT proteins (3 lengths, namely exon 1, N573 and full length) or cocktails of equivalent final protein concentration (Wt + Mut). (C) Same as A, obtained on the same proteins using a (control) TR-FRET immunoassay (2B7/4C9) which does not interrogate the polyQ region and does not detect a temperature- and polyQ dependent conformational change11, 12, demonstrating efficient detection of all proteins. (D) Same as B (ratio TR-FRET signals at 4 °C/20 °C) obtained on the same proteins using a (control) TR-FRET immunoassay (2B7/4C9) which does not interrogate the polyQ region and does not detect a temperature- and polyQ dependent conformational change. In (B and D) values represent means and standard deviations of the means of three independent experiments (two-way ANOVA with Bonferroni’s post-test, degrees of significance are indicated).
