Figure 1.
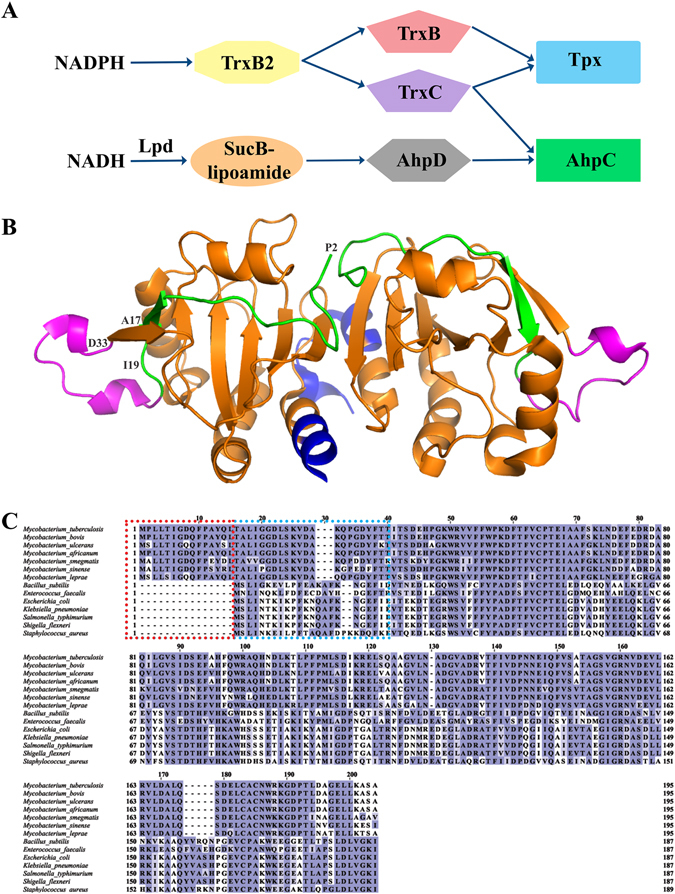
Peroxidatic catalytic pathway of Mycobacterium. (A) AhpC has various disulfide oxidoreductases such as TrxC and the proposed AhpD. In the former, AhpC has been proposed to function with TrxC in the reduction of ROS. In the latter, an orchestra of AhpD, SucB-lipoamide and LpD is necessary to reduce ROS. In both mechanisms, the reduction by either TrxC or AhpD results in the formation of a disulfide bridge along two cysteine residues of AhpC. (B) MtAhpCC176S mutant dimer structure (PDB ID: 2BMX)13. The N-terminal residues 1–15 are highlighted in green. The extra loop (amino acids 23–34) is shown in magenta, and the very C-terminal residues are colored in blue. (C) Multiple sequence alignment of AhpC from M. tuberculosis, M. bovis, M. ulcerans, M. africanum, M. smegmatis, M. sinense, M. leprae, B. subtilis, E. faecalis, E. coli, K. pneumonia, S. typhimurium, S. flexneri and S. aureus using Clustal-Omega30. The stretch of additional residues found in mycobacterial AhpC are highlighted with a red dashed box. The residues deleted for the generation of MbAhpC41–195 are highlighted with a blue dashed box.
