Figure 2.
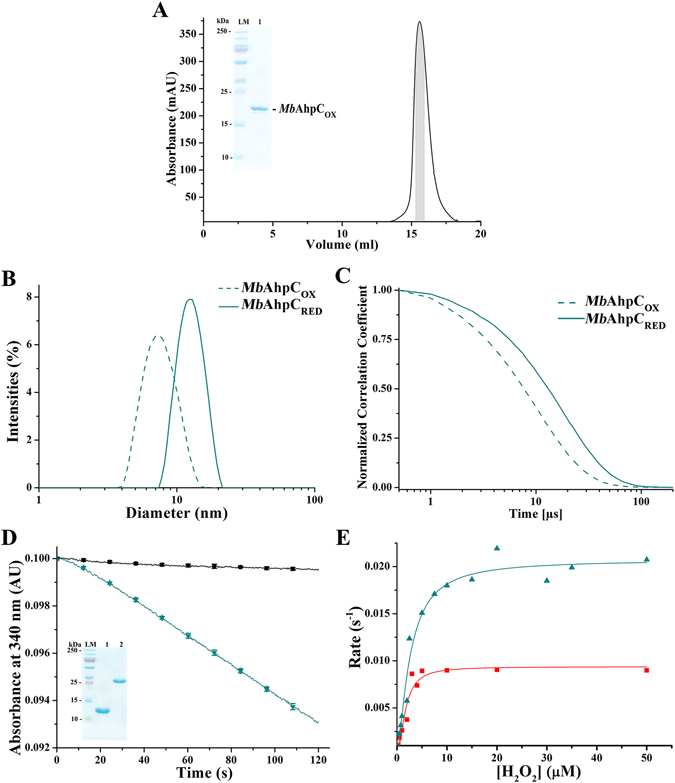
Protein characterization of MbAhpC. (A) The recombinant and oxidized MbAhpC eluted at approximately 16 ml on a Superdex 200 HR 10/30 column and showed high purity on a 17% SDS gel (inset). (B) DLS analysis of the oxidized (green dashed line) and reduced (green solid line) MbAhpC. (C) Normalized correlation function of DLS studies of MbAhpC in 50 mM Tris/HCl, pH 7.5, 200 mM NaCl. A slowed relaxation of the autocorrelation curve in reduced MbAhpC (green solid line) was observed as compared with oxidized MbAhpC (green dashed line). (D) NADPH-oxidation of MbTrxC and MbAhpC. The control experiment was performed in the absence of enzymes and H2O2 (black solid line). The utilized MbTrxC (lane 1) and MbTrxB2 (lane 2) showed high purity on a 17% SDS gel (inset) after size exclusion chromatography purification. The size of MbTrxC is 12,203 Da and MbTrxB2 is 35,643 Da. A significant drop in absorbance was observed due to the presence of wt MbAhpC (green solid line). (E) The Michaelis-Menten plot of MbAhpC and MbAhpCT5A/D8A were performed by fitting data of at least ten concentrations of H2O2 to establish the enzymatic kinetic parameters of wt MbAhpC and MbAhpCT5A/D8A. MbAhpC showed a higher enzymatic efficiency (green line and triangle symbols) as compared to MbAhpCT5A/D8A (red line and square symbols) whereby the reaction approached Vmax rapidly in the mutant as compared to wt MbAhpC.
