Figure 6.
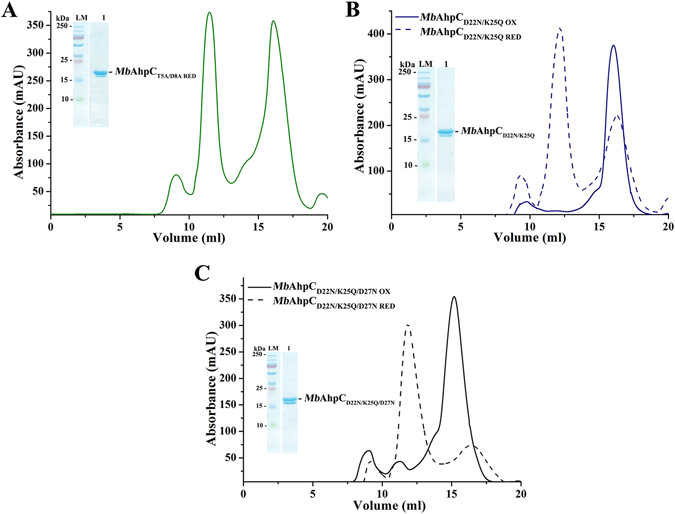
Protein purification of MbAhpC double and triple mutants. (A) The recombinant and reduced MbAhpCT5A/D8A eluted in an equal proportion of oligomer (12 ml) and dimer (16 ml) on a Superdex 200 HR 10/30 column and showed high purity on a 17% SDS gel (inset). The recombinant oxidized and reduced double mutant, (B) MbAhpCD22N/K25Q, and triple mutant, (C) MbAhpCD22N/K25Q/D27N, showed an SEC elution profile resembling wt MbAhpC. Majority of the oxidized recombinant protein (solid blue and black line) eluted at approximately 16 ml while the reduced recombinant protein eluted (dashed blue and black line) at 12 ml. Both reduced recombinant proteins revealed high purity on a 17% SDS gel (inset).
