Figure 9.
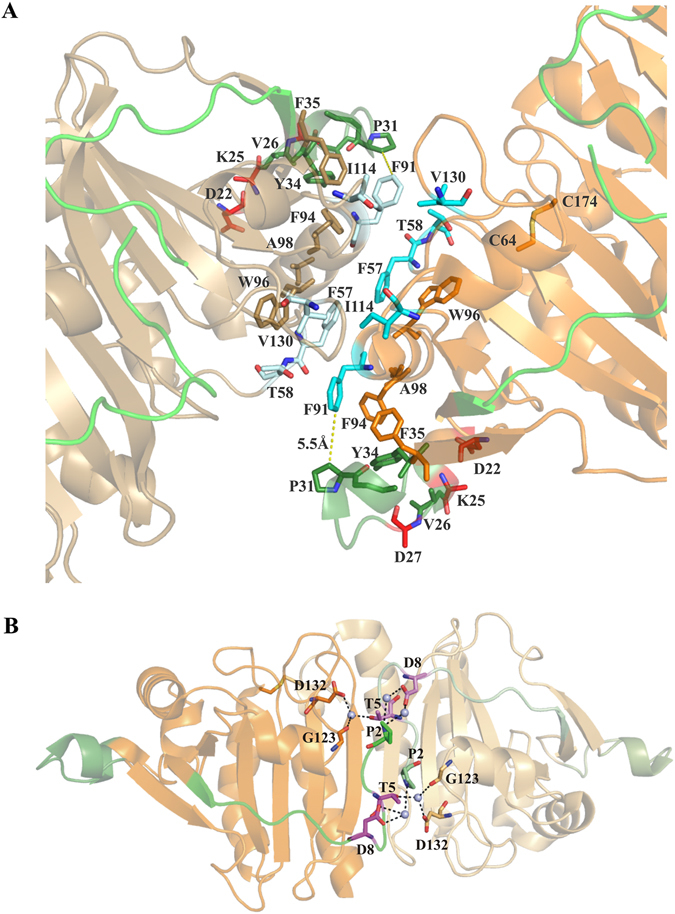
(A) Dimer-dimer interface of the MtAhpCC176S structure (PDB ID: 2BMX)13. The first monomer is shown in orange and the second monomer is shown in sand color. The N-terminal 1–15 residues are shown in green and the extra loop (23–34) in the N-terminal is shown in forest green. The hydrophobic residues F57, T58, F91, I114 and V130 that stabilize the interface are shown in cyan. The mutants D22, K25 and D27 are highlighted in red. (B) Critical residues in the dimer interface of mycobacterial AhpC (PDB ID: 2BMX)13. The mutant residues T5 and D8 are shown in magenta. The subunits are shown in orange with the second in lighter shade. The N-terminal 1–15 residues are shown in green and the extra loop (23–34) in the N-terminal is shown in forest green. The water molecules involved in the water mediated interaction are shown as light blue spheres.
