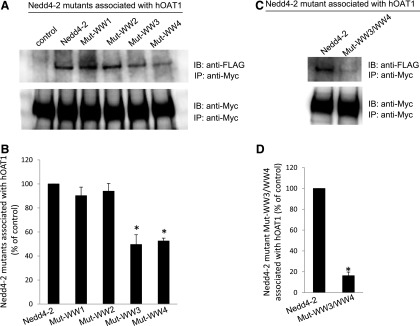
Fig. 6.
Interaction between Nedd4-2 WW domain mutants and hOAT1. (A, top panel) Two amino acid residues in each of the four WW domains of Nedd4-2 were mutated (Mut-WW1: V91W/H93G; Mut-WW2: V283W/H285G; Mut-WW3: I395W/H397G; and Mut-WW4: I446W/H448G). Mutant-transfected cells were lysed, and hOAT1 was then immunoprecipitated (IP) with anti-myc antibody, followed by immunoblotting (IB) with anti-FLAG antibody. Epitope FLAG was tagged to wild-type Nedd4-2 and its mutants for immunodetection of exogenously expressed Nedd4-2 and its mutants. (Bottom panel) The same immunoblot from the top panel was reprobed by anti-myc antibody to determine the amount of hOAT1 IP. (B) Densitometry plot of results from (A, top panel) as well as from other independent experiments. The values are mean ± S.E. (n = 3). *P < 0.05. (C, top panel) WW domain 3 and WW domain 4 were simultaneously mutated (Mut-WW3/WW4). Wild-type Nedd4-2- and mutant Mut-WW3/WW4-transfected cells were lysed, and hOAT1 was then IP with anti-myc antibody, followed by IB with anti-FLAG antibody. (Bottom panel) The same immunoblot from the top panel was reprobed by anti-myc antibody to determine the amount of hOAT1 IP. (D) Densitometry plot of results from (C, top panel) as well as from other independent experiments. The values are mean ± S.E. (n = 3). *P < 0.05.