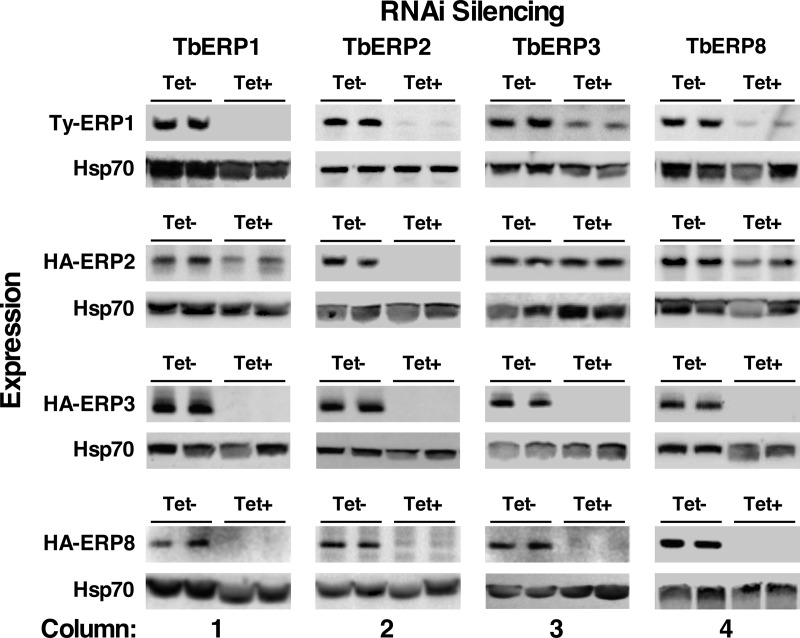
FIG 6 .
TbERP1, TbERP2, TbERP3, and TbERP8 are codependent for stability. The in situ TbERP epitope-tagging constructs were transfected into each of the TbERP RNAi cell lines. Specific dsRNA was induced for 72 h, and whole-cell lysates were prepared from control (Tet-) and induced (Tet+) cells, as indicated in the column labels. Lysates were fractionated by SDS-PAGE (1 × 107 cell equivalents per lane). Membranes were first immunoblotted with anti-TY or anti-HA to evaluate TbERP protein stability under RNAi, as indicated in the row labels. Blots were then stripped and reprobed for cytosolic Hsp70 as a loading control. Two biological replicates are presented (total n = 3). qRT-PCR analyses confirmed that RNAi knockdown is specific, and mRNA levels of opposing tagged TbERP constructs were unaffected, even under significant protein depletion (not shown).