Abstract
Introduction
This critical review aims to summarize published data on limb sparing surgery for extremity soft tissue sarcoma in combination with pre-operative radiotherapy (RT).
Methods
This review is based on peer-reviewed publications using a PubMed search on the MeSH headings “soft tissue sarcoma” AND “preoperative radiotherapy”. Titles and abstracts screened for data including “fraction size AND/OR total dose AND/OR overall treatment time”, “chemotherapy”, “targeted agents AND/OR tyrosine kinase inhibitors”, are collated. Reference lists from some articles have been studied to obtain other pertinent articles. Additional abstracts presented at international sarcoma meetings have been included as well as information on relevant clinical trials available at the ClinicalTrials.gov website.
Results
Data are presented for the conventional regimen of 50–50.4 Gy in 25–28 fractions in 5–6 weeks preoperative external beam RT with respect to the regimen’s local control probability compared to surgery alone, as well as acute and late toxicities. The rationale and outcome data for hypofractionated and/or reduced dose regimens are discussed. Finally, combination schedules with conventional chemotherapy and/or targeted agents are summarized.
Conclusion
Outside the setting of well-designed prospective clinical trials, the conventional 50 Gy in 5–6 weeks schedule should be considered as standard. However, current and future studies addressing alternative fraction size, total dose, overall treatment time and/or combination with chemotherapy or targeted agents may reveal regimens of equal or increased efficacy with reduced late morbidities.
Keywords: Limb Soft Tissue Sarcoma, surgery, preoperativeradiotherapy, combined modality treatment, targeted agents, chemotherapy
Introduction
Limb sparing surgery combined with preoperative external beam radiotherapy (RT) results in high local control rates of at least 85–90% in patients with extremity soft tissue sarcomas (ESTS) resected with negative margins [1–3] and, in conjunction with limb conservation surgical approaches, has widely replaced the need for amputations [4]. Traditionally, the prescription dose for preoperative RT is 50 Gy delivered in 1.8–2 Gy fractions over five weeks and for post-operative RT is 60–66 Gy delivered in 1.8–2 Gy fractions over six to seven weeks. The surgical community has not yet widely adopted referral of ESTS patients for preoperative RT, basing their reluctance upon the higher rate of wound complications and imposed delay to definitive surgery. This review panel acknowledges these points. However, the (sometimes severe) acute complications are generally of a temporary nature. Conversely, the potential decreased functional morbidity, which is more prevalent and significant following postoperative RT compared to preoperative RT, is, typically permanent and frequently progressive in severity. For this reason, and for the possibility of schedule modification, the remainder of this manuscript will focus on preoperative RT only. Although endpoints for local control and overall survival do not differ for pre-versus postoperative RT, the toxicity parameters differ and these toxicities may be significant for some patients. After postoperative RT, fewer acute wound complications are seen (17% versus 35%) [1]. However, after prolonged follow up, more late toxicities such as fibrosis, arthrosis and edema resulting in diminished functional outcome are reported [5]. Anatomic site also plays an important part in the toxicity profile, since patients with upper extremity lesions are unlikely to suffer from the same rate of wound complications following preoperative RT compared to those with lower extremity lesions [1, 6].
In patients with negative margins after preoperative RT, an excellent local control outcome can be anticipated. However, local control rates may drop to as low as 62% at 5 years when positive resection margins after preoperative RT are achieved [7–9]. Unfortunately, the addition of a postoperative boost in this setting has not been shown to improve local control outcomes [9–10]. Furthermore, not all clinical settings of positive surgical margins are the same. They should be clearly defined and analyzed separately. O’Donnell et al [8], were able to retrieve 169 patients, from their prospective sarcoma database, all with positive resection margins, treated between 1986 and 2009. These cases were stratified into 3 groups, each representing a specific clinical scenario: those with a critical structure positive margin (e.g. major nerve, blood vessel, or bone), those with a tumor bed resection positive margin, and those with an unexpected positive margin during primary resection. The 5-year local recurrence-free survival rates were 85.4%, 78.9%, and 63.4% respectively, suggesting, that sparing of adjacent critical structures in this setting is relatively safe and contributes to improved functional outcomes. Therefore, especially when positive margins are planned or expected, these patients could be considered for innovative strategies, such as dose painting (i.e. focal dose escalation) and/or radiosensitization with novel agents. Furthermore, it should be acknowledged, that for those cases that do occur, the site of local recurrence is usually within the high dose irradiated volume [12–15].
Novel treatment strategies to improve outcome of patients presenting with localized ESTS, aiming to maintain or increase local control probability while diminishing early and late toxicity, are warranted. Furthermore, ESTS consists of a group of diseases which includes many histological subtypes with specific characteristics reflective of underlying differences in biology, genetics, clinical behavior and/or sensitivity to both chemotherapy and radiotherapy. Accordingly, it is improbable that all these entities will benefit from a single uniform regimen.
Several additional issues merit consideration: (1) the radiation fractionation including fraction size, total dose and overall treatment time, as well as (2) the opportunity to combine radiotherapy with conventional chemotherapy and/or targeted agents in addition to (3) the possibility that different treatment schedules may be appropriate for different histological subtypes. A consensus statement for sarcoma brachytherapy has been recently published [16]. The role of brachytherapy is beyond the scope of this review article.
Methodology
This review is based on peer-reviewed publications using a PubMed search on the MeSH headings “soft tissue sarcoma” AND “preoperative radiotherapy”. Titles and abstracts screened for data including “fraction size AND/OR total dose AND/OR overall treatment time”, “chemotherapy”, “targeted agents AND/OR tyrosine kinase inhibitors”, were collated. Reference lists from some articles were studied to obtain other pertinent articles. Additional abstracts presented at international sarcoma meetings were included. Information on relevant clinical trials was obtained from the ClinicalTrials.gov website.
Current knowledge on fraction size, total dose and overall treatment time
For preoperative RT, the prescription of 50 Gy in 1.8–2 Gy once-daily fractions over 5–6 weeks, is the current standard schedule [2]. Both the NCCN [17] and ESMO guidelines [18] suggest combining conservative surgery and RT for most cases of intermediate or high grade ESTS.
However, in selected patients, omission of RT could be considered [19–21]. In particular, cases where the closest resection margin is more than 1cm are likely associated with high local control rates even without RT. Pisters et al [19] analyzed a carefully selected population of 88 patients with T1 sarcomas. The 10 year estimated cumulative local recurrence rate without RT was 16.2% for the entire group and 10.6% for the subgroup after R0 surgery. Baldini and co-workers [20] have reported on 74 patients, with sarcomas of a median size of 4 cm (range 0.5–31 cm) treated by surgery only. They found a 10-year local failure rate of 13% when the surgical margins were < 1.0 cm but no local failures when the margins were ≥ 1cm. The Memorial Sloan Kettering Cancer Center (MSKCC) sarcoma database was used to develop a nomogram based on clinicopathologic factors of 684 patients to quantify the risk of local recurrence after limb sparing surgery without adjuvant RT [22]. The prediction tool is available on their website. Since this nomogram was developed from a retrospective series assessing a group of patients who were selected by their clinician not to receive radiation, it may harbor unrecognized selection biases. It may well be that the true risk of local recurrence in an unselected group of ESTS patients treated with surgery alone is underestimated by the nomogram. Conversely, in experienced multidisciplinary sarcoma team management, the most unfavorable subgroup (age above 50 years, sarcomas larger than 5 cm, resected with close or positive margins, and unfavorable histological subtypes) exhibits a local control rate without RT of 53% at 5 years (see also Figure 1). Local recurrence after 5 years is rare, so this percentage can be considered a true reflection of clinical practice. For these 53% of patients with durable local control following surgery alone, any form of RT would have been overtreatment. This rate of local control after surgery alone should be considered alongside the “no-RT” arms of the 2 available randomized studies reported by Pisters et al [23] (69% at 5 years) and Yang and colleagues [3, 24] (68–78% at 10–20 years dependent upon histological grade). Furthermore, 10–15% of patients recur locally despite the use of combined surgery and RT [1, 2]. This leaves a potential subgroup of approximately 30–40% of patients who appear to truly benefit from the addition of RT to limb sparing surgery. This percentage is clinically significant and similar to that seen after breast conserving surgery, especially in younger women [25]. It is important to note, that randomized data do not support treatment with surgery alone and the omission of RT for most patients remains investigational. Also, criteria for RT omission need further definition and may include factors such as: tumor of T1 size, superficially located, resected with wide (>1 cm) negative margins, specific histological subtypes (like atypical lipomatous tumors), and location such that a local recurrence would be amenable to salvage surgery.
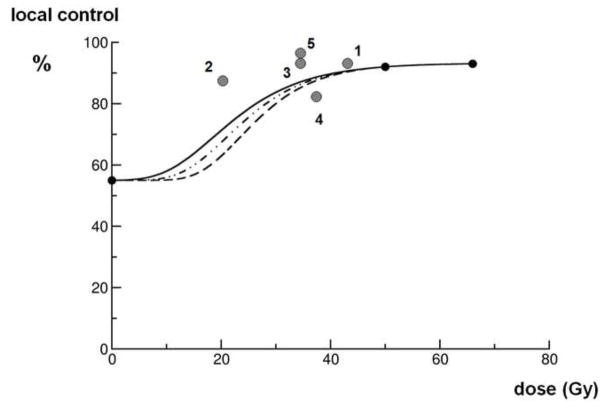
Figure 1.
A hypothetical local control probability curve, for simplification, calculated by: S = exp(− [αD + βD2]). In this graph, at 0 Gy the most unfavorable subgroup of patients (age above 50 years, sarcomas larger than 5 cm, resected with close or positive margins, and unfavorable histological subtypes as outlined in the text) in the Memorial Sloan Kettering Cancer Center (MSKCC) nomogram [22] is chosen and at 50 Gy (preoperative) and at 66 Gy (postoperative) the outcomes of the NCIC SR-2 trial [1]. The three lines come forth from low to high α/β ratios calculations. The grey dots numbers 1, 2 and 3 represent the three consecutive Eilber studies [26, 27], number 4 comes from the Kosela study [31], and number 5 represents Temple’s data [28]. The biological equivalent dose (BED) of these dots are calculated assuming an α/β ratio of 4 Gy (5 × 3.5 Gy equals BED of 21,875 Gy, 8 × 3.5 Gy equals a BED of 35 Gy, 10 × 3.5 Gy equals a BED of 43,75 Gy, 5 × 5 Gy equals a BED of 37,5Gy, and 10 × 3 Gy equals a BED of 35 Gy). All points must be skewed to the left if these α/β ratios are higher than 4 Gy. All data derived from clinical studies and observations fairly match the calculated curves.
An alternative dose fractionation approach, employed 3–4 decades ago in the early period of limb preservation, is represented by the studies of Eilber and colleagues [26, 27]. In three consecutive time periods, three different preoperative regimens all containing intra-arterial or intravenous adriamycin were tested. From 1974–1981, 77 patients received 10 × 3.5 Gy, from 1981–1984, 137 patients received 5 × 3.5 Gy and from 1984–1987, 112 patients received 8 × 3.5 Gy. In all studies after 1987, either cisplatin or ifosfamide were added, but the RT prescription remained unchanged at 8 × 3.5 Gy. The local failure rate in the first era was 5% at 8 years, in the second era 12% at 4 years, and in the last era 5% at 2 years. However, no long-term follow up data on late functional sequelae are available from these 3 studies. Temple and colleagues have also combined intra-arterial or intravenous adriamycin with preoperative RT; their regimen was 10 × 3 Gy [28]. By further reducing the fraction size from 3.5 Gy to 3 Gy, they were able to reduce the wound complication rate to 15%, while maintaining local control at 97% at 5 years follow up.
Although the α/β ratio for the different sarcoma subtypes is unknown, it is possible that the value is below 10 Gy [29]. Figure 1 shows a hypothetical local control probability curve as a function of biological equivalent doses (BED) in conventional 2 Gy fractionation assuming an α/β ratio of 4 Gy. In a more conventional calculation with a ratio of 10 Gy, the data points represented by grey dots would be skewed to the left. Thus, the BED of all Eilber’s regimens are below the reference schedule of 50 Gy in 2 Gy fractions. It should be noted, that decreasing the RT dose as low as 5 × 3.5 Gy was unacceptable because it resulted in a higher local recurrence rate.
An alternative approach, explored in the RTOG 9514 study, was to reduce the total radiotherapy dose intensity while combining RT with chemotherapy [30]. Here the investigators reduced the dose of RT from 50 Gy to 44 Gy in 2 split series of 11 × 2 Gy sequenced with chemotherapy (see further details below). R0 resections were achieved in 91% of cases. At 3 years, the local control rate was 90%, but the toxicity profile for this combined chemotherapy and RT approach was significant as discussed below. Late functional outcome data from this study have not been reported.
Another novel approach to decrease radiotherapy dose is represented by a report from the Polish Sarcoma Study Group. Kosela-Paterczyk and colleagues performed a prospective phase II clinical trial which accrued 272 patients and investigated a dose of 5 × 5 Gy followed by surgery three to seven days after completion of RT. After a median follow-up of 35 months, the estimated 5 year local failure rate was 19% [31], which may be on the lower level of acceptable local control.
Finally, reports for myxoid liposarcomas (MLS) have consistently shown exquisite radiation sensitivity, characterized by a marked tumor volume reduction during radiotherapy and excellent local control rates [32–34]. After surgery, the resection specimens frequently show a fibrotic myxoid stroma containing, nonlipogenic, hyalinized structures. Gross evidence of tumor necrosis is uncommon, but often only a few (if any) visible tumor cells remain on microscopic examination. Furthermore, the specimens show a substantial effect on medium-sized arterioles with intimal hypertrophy and parietal thrombus formation. The classic delicate crow’s feet capillary vasculature can still be identified [35]. A dose reduction to 18 × 2 Gy for MLS is now being investigated in an international multi-center prospective phase II clinical trial (ClinicalTrials. Gov Identifier: NCT02106312). If excellent local control can be maintained with this reduced dose, and both wound complications and long-term toxicities are also reduced, this would result in a significant advantage for patients, albeit potentially singularly applicable to this sarcoma subtype with unusually high response and sensitivity to radiotherapy. In order to compare the published data, recalculating the Eilber schedules to a 2 Gy per fraction regimen is necessary. The regimen of 8 × 3.5 Gy = 28 Gy would result in a BED of 31.5 Gy with a conventional α/β ratio of 10 Gy, a BED of 35 Gy with a reasonable α/β ratio of 4 Gy or a BED of 38.5 Gy with an extremely low α/β ratio of 2 Gy. Reviewing all data on radiation dose, it is reasonable to assume that a dose response relationship exists for local control below 28 Gy [27] in 8 fractions of 3.5 Gy. In the preoperative setting, this dose response relationship between 28 Gy and 50 Gy is uncertain and may well be marginal (see also Figures 1 and 2). On the other hand, it is also reasonable to assume that acute wound complications are related to RT dose and volume [6, 26, 27, 36–38]. The impact of fractionation on late functional outcome has yet to be fully explored. The mature results of the relatively extreme hypofractionated Polish strategy will provide valuable insight in the relationship between hypofractionation in combination with a dose reduction on late radiation effects.
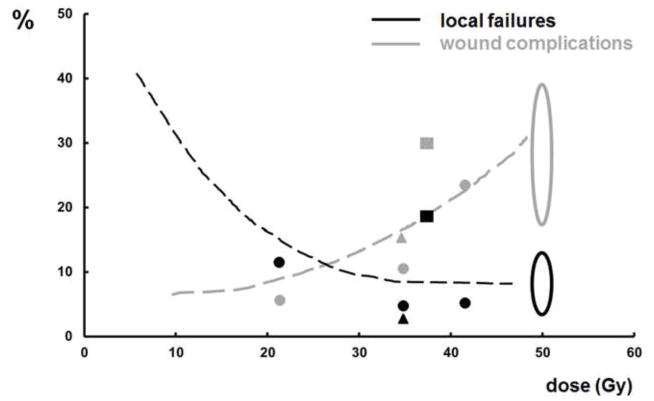
Figure 2.
In Figure 2 two data sets on local control (shapes in black) and wound complications (shapes in grey) are combined. The black dots represent the local failures in the Eilber studies [26, 27] (see also Figure 1). The black oval summarizes the projected 5 years local failure probability from Table 2. The black striped line connects these outcome data and intends to intersect the y-axis at a local control achieved by surgery only as described in Table 2. The grey line, dots and oval represents the wound complication rate as a function of BED from the same references. The black and grey squares represent, respectively, the local failure- and wound complication probability as published by Kosela et al [31]. Finally, the black and grey triangles represent, respectively, the local failure- and wound complication probability as reported by Temple et al [28].
The data on the fractionation characteristics of all the radiotherapy regimens mentioned above are summarized (see Figures 1 & 2 and Table 1), including both non-randomized and randomized trial results to appreciate the diverse fractionation schedules evaluated over the last 30 years. Figure 1 depicts a hypothetical local control probability as a function of dose calculated by: S = exp(− [αD + βD2]). In this graph, at 0 Gy the most unfavorable subgroup of patients from the MSKCC nomogram [22] is chosen and at 50 Gy and 66 Gy the data points represent the outcomes of the NCIC SR-2 trial [1]. The clinical trials data between 0 and 50 Gy match the calculated curves. Figure 2 suggests that there may be a threshold dose for local control not at, but below 50 Gy. The extrapolated curve for wound complications, however, seems to exhibit a more linear to exponential dose response relationship without a threshold value. Obviously, the quality of surgery also has to be considered in the interpretation of local control and toxicity date.
Table 1.
Retrospective and Randomized data on surgery alone and (neo-) adjuvant RT in ESTS. This table summarizes published data on radiotherapy only with respect to the radical resectability R0, wound complications and local control. The - mark means no data are available on this issue in the full paper. In the nomogram by Cahlon [22], the most unfavorable patient characteristics (for details see text) were chosen. In the study by Yang [24] 141 patients were randomized; 91 with high grade sarcomas and 50 with low grade histologies, 70 received radiotherapy and 71 did not.
| Retrospective and Randomized data on Surgery Alone and (neo-) adjuvant RT plus Surgery in ESTS | ||||||
|---|---|---|---|---|---|---|
| Author/Reference | n | RT regimen | CT regimen | R0 resections | Wound complications | Local control (@ X years) |
| Cahlon [22] | 684 | 0 Gy (surgery only) | none | – | – | 53% @ 5 yrs |
| Pisters [19] | 88 | 0 Gy (surgery only) | none | 84% | – | 84% @ 10 yrs |
| Baldini [20] | 74 | 0 Gy (surgery only) | none | 92% | – | 93% @ 10 yrs |
| Rosenberg [4] | Randomized trial n = 16 versus 27 |
0 Gy (amputation) | ACM (all cases) | 100% | – | 100% @ 4.7 yrs |
| 50 + 10–20 Gy | 85% | 85% @ 4.7 yrs | ||||
| Pisters [23] | Randomized trial n = 78 versus 86 |
0 Gy (surgery only) | none | 82% | 10% | 69% @ 5 yrs |
| 42 – 46 Gy brachytherapy | none | 48% (direct afterloading) 14% (delayed afterloading) | 82% @ 5 yrs | |||
| Yang [24] | Randomized trial n = 141 (for details see Legend) |
0 Gy (surgery only) | Adjuvant 5 times AC for all high grade STS | – | Trial randomized postoperative RT only | In high grade STS 78% versus 100% @ 10 yrs In low grade STS 68% versus 95% @ 10 yrs |
| 45 + 18 Gy postoperative | – | |||||
| O’Sullivan [1] | Randomized trial n = 94 versus 96 |
50 Gy preoperative | none | 84% | 35% | 92% @ 3.3 yrs |
| 50 + 16 Gy postoperative | none | 86% | 17% | 93% @ 3.3 yrs | ||
Abbreviations: RT = radiotherapy, ESTS = extremity soft tissue sarcoma, n = number of cases, CT = chemotherapy, AC = doxorubicin and cyclophosphamide, ACM = doxorubicin, cyclophosphamide and methotrexate.
Current knowledge pertaining to the combination of RT and conventional chemotherapy and/or targeted agents for STS
For many epithelial tumors (e.g. rectal-, head and neck-, lung and esophageal cancer), concurrent treatment with systemic agents and external beam RT frequently results in an increased local control probability, which sometimes translates into increased overall survival benefit, and has thus become part of the standard of care. The disadvantage of such approaches is that they are generally associated with an increased, usually temporary, though sometimes severe acute toxicity profile. These toxicities vary based on the tumor site and the specific systemic agents delivered.
This combined modality treatment toxicity may be severe in certain patients. Nonetheless, these data suggest that it may be worthwhile to explore combinations of RT plus systemic agents, including radiosensitizers in ESTS especially in patient subgroups at high risk for local and/or distant failure such as those planned to have positive surgical margins. Obviously, careful long-term observation of late functional outcome is required in the design of such new combinations. It is presently unclear how to best measure the clinical benefit of induction treatment for localized ESTS. Late outcomes such as local control, quality of life and overall survival can be considered as robust endpoints, but they take years to observe. Surrogate early end-points provide an alternative assessment strategy, represented by outcomes such as the pathological evaluation of the resection specimen, wound complications, and potential signals from sophisticated imaging techniques [39–41]. All need to be incorporated into prospective clinical studies to be validated. For this section, the combined modality regimens will be compared to radiotherapy only, especially with respect to the induction of necrosis in the resection specimens, local control and wound healing problems. In the literature, a generally accepted definition of a pathological complete remission (pCR) is represented by greater than or equal to 99–100% necrosis (or less than or equal to 1% residual visible tumor cells), whereas a near pCR can be defined as greater than or equal to 95% necrosis. Canter [42] and Shah [43] demonstrated, that a (near) pCR can be appreciated in only 8–10% of cases following RT alone to 50 Gy in 2 Gy fractions. Nevertheless, the true prognostic significance of treatment-induced pathologic necrosis in ESTS after neoadjuvant therapy has yet to be determined [44].
In the following sections, research focusing on combinations of RT with conventional chemotherapy as well as combinations with more modern targeted agents are summarized (see Table 2).
Table 2.
Preoperative RT in ESTS either with or without sensitizers. This table summarizes published data on radiotherapy only or in combination with conventional chemotherapy or targeted agents with respect to the percentage of induced necrosis, the radical resectability, wound complications and local control. The - mark means no data are available on this issue in the full paper.
| Preoperative RT in ESTS either with or without sensitizers. | ||||||||
|---|---|---|---|---|---|---|---|---|
| Setting | Author (Reference) | n | RT regimen | CT regimen | (near) pCR | R0 resections | Wound complications | Local control (@ X years) |
| RT only | Canter [42] | 25 | 25 × 2 Gy | – | 8% | 84% | 28% | 100% @ 3 yrs |
| Shah [43] | 30 | 25 × 2 Gy | – | 10% | – | 23% | 100% @ 5 yrs | |
| Kosela [31] | 272 | 5 × 5 Gy | – | – | 79% | 32% | 81% @ 3 yrs | |
| RT + conventional chemotherapy | Eilber ‘74–‘81 [26, 27] | 77 | 10 × 3.5 Gy | Adria | 12% | – | 23% | 95% @ 8 yrs |
| Eilber ’81–‘84 [26, 27] | 137 | 5 × 3.5 Gy | Adria | 4% | – | 5% | 88% @ 4 yrs | |
| Eilber ’84–‘87 [26, 27] | 112 | 8 × 3.5 Gy | Adria | 6% | – | 10% | 95% @ 2 yrs | |
| Temple [28] | 42 | 10 × 3 Gy | Adria | – | – | 15% @ | 97% @ 5 yrs | |
| Kraybill [30] | 59 | split course 2 times 11 × 2 Gy | MAID | 27% | 91% | 11% | 91% @ 3 yrs | |
| Ryan [50] | 25 | 8 × 3.5 Gy | Adria/Ifos | 40% | 88% | 20% | 88% @ 2 yrs | |
| MacDermed [49] | 34 | 8 × 3.5 Gy | Ifos | 11.8% | 100% | 17% | 89% @ 5 yrs | |
| RT + targeted agents | Yoon [58] | 20 | 28 × 1.8 Gy | Avastin | 20% | – | 20% | 95% @ 2 yrs |
| Canter [59] | 8 | 25 × 2 Gy | Sorafenib | 38% | 75% | 38% | 100% @ 3 yrs | |
| Meyer [41] | 16 | 8 × 3.5 Gy | Sorafenib * | 44% | 94% | 38% | 100% @ 2 yrs | |
| Lewin [60] | 9 | 28 × 1.8 Gy | Sunitinib | # | – | – | # | |
| Haas [61] | 11 | 25 × 2 Gy | Pazopanib | 40% | – | 20% | 91% @ 2 yrs | |
| RT + intratumoral nanoparticles | Bonvalot [65] | 20 | 25 × 2 Gy | Hafnium oxide nanoparticles | 18% | – | 5% | – |
Abbreviations: RT = radiotherapy, ESTS = extremity soft tissue sarcoma, CT = chemotherapy, pCR = pathological complete remission, n = number of cases, Adria = doxorubicine, Ifos = ifosfamide, MAID = mesna, doxorubicin, ifosfamide, and dacarbazine. ASCO = the American Society of Clinical Oncology, CTOS = the Connective Tissue Oncology Society.
in this study Sorafenib was combined with epiribucin and ifosfamide.
in this study, the pathological response was described as the median percentage of necrosis (see text), the 2-years progression-free survival was 44%.
in this study, wound complications were scored as either “major” (wound necrosis secondary to thrombosis of reconstructed artery) in 2.5% of cases or “minor” in 12.5%.
Conventional chemotherapy combined with RT in preoperative STS management
The previously mentioned RTOG 9514 trial (discussed in the context of its RT dose reduction) investigated the so called “MAID” regimen [30]: mesna, doxorubicin, ifosfamide, and dacarbazine chemotherapy, interdigitated with preoperative split course RT and three cycles of postoperative chemotherapy. While not a true concurrent radiosensitization approach, it merits comments in this section. The regimen was toxic with 83% grade IV and 5% grade V toxicities; in part, this was because the RT fields extended 9 cm above and below gross disease, as well as the fact that the ifosfamide dose was 2500 mg/m2 which was higher than that explored in a prior pilot study [45]. Nonetheless, this combination appeared to achieve a pCR in 27% of the evaluable patients. Of course, it is difficult to determine if the pCR comes as a consequence of radiotherapy, chemotherapy or the interdigitated combination of both. Reports with longer follow-up of this study have shown a significant survival benefit for those treated with chemotherapy [46]. However, the entire study population also experienced a relatively high local failure rate of 22.2% at 5 years, a relatively high amputation rate of 9.4% (including all amputation for any cause including unsuitability for limb-sparing surgery at the time of assessment after induction chemoradiation) and 2 cases of acute myelogenous leukemia [47].
Ifosfamide-based regimens have been investigated in retroperitoneal sarcomas [48] and in ESTS [49]. MacDermed et al combined the 8 × 3.5 Gy schedule with concurrent ifosfamide (2.5 g/m2 per day for 5 days) albeit with higher than conventional doses per fraction. They reported a pCR in 11.8% of cases, with R0 resections performed in all cases, and a 5 year local control rate of 89% [49].
Ryan et al [50] combined the same regimen of 8 × 3.5 Gy regimen with epirubicin 30 mg/m2 per day and ifosfamide at a dose of 2.5 g/m2 per day, both on days 1 to 4, in ESTS and body wall sarcoma patients. These agents are among the more effective drugs in sarcoma. Though this regimen was toxic, a (near) pCR was found in 40% of all resection specimens.
Drugs that possibly deserve additional attention in the setting of STS are gemcitabine and temozolomide due to their proven radiation sensitization, but data for these agents are scarce [51]. Furthermore, apart from the use of gemcitabine as treatment for metastatic leiomyosarcomas, data showing single agent efficacy are lacking [52]. Reviewing (neo-)adjuvant chemotherapy trials (e.g. the Italian/Spanish [53], the EORTC 62931 [54] and the RTOG 9514 [30] studies), it can be concluded that, delaying RT in these trials had no adverse effect on the observed local control rate, but delivery of chemotherapy did not negate the necessity for RT.
Targeted agents combined with RT in preoperative STS management
From a biological point of view, studies combining targeted agents with RT are very appealing. Neovascularization and angiogenesis are fundamental mechanisms in tumor initiation, promotion, and the acquisition of a metastatic phenotype [55]. Overexpression of vascular endothelial growth factor (VEGF) and its receptors have been observed as neoplastic phenomena. Also STS have been shown to overexpress angiogenic factors in both tumor tissue and serum, thereby underpinning the exploration of anti-angiogenic compounds in the treatment of STS [56]. In addition, early stage clinical trials suggest that the combination of RT and antiangiogenic agents may exhibit a synergistic effect [57]. Radiosensitization could be both clinically and biologically significant in STS since complete and near-complete pathologic response have been associated with improved oncologic outcomes in some series of STS patients treated with neoadjuvant therapy [42] although the relationship is less clear in other series [44]. It should be expected, that combining RT with targeted agents may result both in increased toxicity within the radiation volumes as well as the known systemic side effects of the compounds by themselves (i.e. alterations in thyroid- and liver function tests, blood pressure etc.). Research in this area is outlined below and summarized in Table 2.
Yoon and colleagues [58] combined 28 × 1.8 Gy with bevacizumab in a preoperative setting. This regimen resulted in ≥80% necrosis in 45% of tumors, 20% grade III systemic toxicities (hypertension and altered liver function tests), 75% R0 resections and 20% major wound complications. At a median follow up of 24 months, there were no local recurrences among the 13 ESTS patients (while only 1 out of 6 patients with a retroperitoneal/pelvic sarcoma had a local recurrence, which is of interest, because this site is known for its high local failure rate).
Canter et al [59] investigated sorafenib combined with 25 × 2 Gy in a phase I trial where three dose levels were planned. The maximal tolerated dose was reached at the second level (200mg + 400mg daily). At this second dose level, grade 3 toxicities in 80% of cases were observed including skin rash that prevented drug re-introduction in 2 of 5 patients, anemia and supraventricular tachycardia in 1 of 5 cases, and a perirectal abscess in one patient. Major wound complications (grade 3) were observed in 3 of 8 cases while 6 of 8 cases underwent R0 resections. All patients exhibited local control at a median follow up of 3 years. The authors suggest that further investigation of the first dose level that employed twice daily 200mg Sorafenib is warranted.
Meyer and colleagues [41] combined sorafenib with 8 × 3.5 Gy of preoperative epiribucin and ifosfamide-based chemoradiation for high risk extremity soft-tissue sarcomas. A parallel correlative study with dynamic contrast enhanced (DCE) MRI was performed to assess response to treatment. Patients received 3 cycles of epirubicin and ifosfamide pre-operatively and 3 cycles post-operatively. Epirubicin was omitted during radiotherapy. Sixteen of eighteen patients were evaluable with a maximum tolerated dose of sorafenib at 400mg once daily. A high incidence of febrile neutropenia (~50%) was reported. Forty four percent of patients demonstrated ≥ 95% necrosis. DCE-MRI after 2 weeks of sorafenib correlated with histologic response.
A note of caution was presented by Lewin and colleagues [60] on the combination of 28 × 1.8 Gy with sunitinib. Here, even after dose de-escalation of sunitinib, they observed an unexpected 44% grade 3+ hepatotoxicity rate and an overall grade 3+ toxicity rate of 78%. Furthermore, a higher local failure rate (HR: 8.1; p = 0.004) was apparent in patients receiving sunitinib. However, the combination of sunitinib plus RT led to an almost doubling of the median tumor necrosis percentage (40%, range 5–100%, versus 75%, range 1–95%) as compared to RT alone.
Finally, it has been suggested, that a combination of 25 × 2 Gy plus dose-escalated pazopanib seems safe up to the highest pazopanib dose level of once daily 800 mg [61]. However, in this study the grade 3+ hepatotoxicity rate was unexpectedly high at 27%. In 40% of the resection specimens a pathological (near) complete remission could be appreciated.
These receptor tyrosine kinase inhibitor (RTKI) based studies are encouraging but they need to be confirmed in larger cohorts with longer follow up. Warnings have come forth from animal experiments showing a more invasive and metastatic potential after administration of RTKI’s. To date, in humans, there are no available data concerning rapid metastatic disease after RTKI application in the adjuvant setting. There are also no available data in humans, on rapid disease progression after RTKI withdrawal in metastatic patients [62, 63].
Preliminary results of other phase I trials have been presented in abstract form: sunitinib in combination with 28 × 1.8 Gy [64], and hafniumoxide nanoparticles (NBTXR3, intended to enhance the RT effect by local electron deposits) in combination with 25 × 2 Gy [65]. Because of the promising results observed with intra-tumoral injection of NBTXR3 nanoparticles just prior to preoperative RT followed by surgery in a phase I trial (showing a median percentage of residual malignant visible cells of 25%), a phase II/III trial has just started comparing preoperative radiotherapy to 50 Gy to the same RT schedule combined with intra-tumoral NBTXR3 (Trial Identifier NCT02379845). Longer follow up and full manuscripts of these regimens are awaited. Furthermore, the use of the pathological response as a surrogate point of local control or outcome needs to be evaluated in future studies.
Discussion
The sarcoma scientific community should engage in a re-evaluation and optimization of the conventionally fractionated preoperative RT schedule of 25 × 2 Gy. Modifications to this regimen may be challenging because of some systematic barriers faced by sarcoma researchers. Specifically as an “orphan disease”, sarcoma research to address translational questions and/or conduct studies has always been more challenging to fund at the grant competition level as well as through industry when compared to common cancers. However, with clear scientific methodology, opportunities for treatment adjustment would exist through investigations addressing both the schedule itself and possible combination with radiation sensitizers. In addition to potential improvement in oncologic outcome (especially after R1 resections and/or in histological subtypes more prone to local relapse such as myxofibrosarcoma and malignant peripheral nerve sheath tumor; [66–69]), these combinations may also offer opportunities to decrease the RT dose for patients where local control would be anticipated to be high but there is concern about the potential toxicity of radiation. Although 25 × 2 Gy remains standard for preoperative management of ESTS [1, 2], this regimen is not based upon robust evidence emanating from randomized trials comparing different preoperative RT dose levels. Although, the Polish 5 × 5 Gy schedule and the MLS reduced dose study are examples of completed or ongoing investigations respectively, they remain phase II experiences that need appropriate validation while also recognizing, as mentioned earlier, that the former study reported a lower than expected local control while the control rate for the latter study is not yet reported. In the treatment of breast cancer, conventional 2 Gy fractionation regimens have largely been replaced by hypofractionated schedules with adequate total dose for the fractionation chosen. This may be a reasonable approach for the treatment of many types of STS as well [70]. Delayed wound healing is a serious adverse event after preoperative RT. This risk is probably partly related to patient and tumor characteristics (e.g. obesity, diabetes, smoking habits and the location of the sarcoma especially those in proximity to major neurovascular structures in the lower extremities), as well as radiotherapy parameters such as total dose, fraction size, treatment volume, skin flap sparing and sophisticated RT techniques [6, 13, 36–38, 71]. The approach of a reduced preoperative RT dose in combination with sensitizing agents could be a great step forward if such combinations could maintain or improve local control in association with a reduction in perioperative and long-term morbidity (see figure 2), ideally improving late functional outcome and quality of life for these patients. The toxicity profile and costs of such agents should be balanced against the desired gain in oncological outcome parameters. Well-designed randomized phase III clinical trials are the best tool to evaluate proposed new regimens. Unfortunately, in the setting of rare diseases like sarcomas, this may be problematic. New approaches to address this challenge should be explored. For example, trials based upon modern Bayesian principles [72], such as the reduced dose MLS trial, may provide alternative means to acquire reasonable evidence to guide future local management in this rare malignancy.
Acknowledgments
The authors acknowledge Dr. A.L. Wolf from the Department of Medical Physics, The Netherlands Cancer Institute, Amsterdam for her help in the design of Figure 1.
Footnotes
Conflicts of interest: all authors: none, except RH receiving research grants, though unrelated to the topic of this manuscript
Meeting presentation: not done
Conflict of Interest Statement.
all authors: none, except RH receiving research grants, though these grants and the supplying sponsors are unrelated to the topic of this manuscript.
Grant or other financial support: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft tissue sarcoma of the limbs: a randomised trial. Lancet. 2002;359:2235–41. doi: 10.1016/S0140-6736(02)09292-9. [DOI] [PubMed] [Google Scholar]
- 2.Haas RL, Delaney TF, O’Sullivan B, et al. Radiotherapy for Management of Extremity Soft Tissue Sarcomas: Why, When, and Where? Int J Radiat Oncol Biol Phys. 2012;84:572–80. doi: 10.1016/j.ijrobp.2012.01.062. [DOI] [PubMed] [Google Scholar]
- 3.Beane JD, Yang JC, White D, et al. Efficacy of adjuvant radiation therapy in the treatment of soft tissue sarcoma of the extremity: 20-year follow-up of a randomized prospective trial. Ann Surg Oncol. 2014;21:2484–9. doi: 10.1245/s10434-014-3732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196:305–15. doi: 10.1097/00000658-198209000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis AM, Sennik S, Griffin AM, et al. Predictors of functional outcomes following limb salvage surgery for lower-extremity soft tissue sarcoma. J Surg Oncol. 2000;73:206–11. doi: 10.1002/(sici)1096-9098(200004)73:4<206::aid-jso4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Baldini EH, Lapidus MR, Wang Q, et al. Predictors for major wound complications following preoperative radiotherapy and surgery for soft-tissue sarcoma of the extremities and trunk: importance of tumor proximity to skin surface. Ann Surg Oncol. 2013;20:1494–9. doi: 10.1245/s10434-012-2797-1. [DOI] [PubMed] [Google Scholar]
- 7.Tanabe KK, Pollock RE, Ellis LM, et al. Influence of surgical margins on outcome in patients with preoperatively irradiated extremity soft tissue sarcomas. Cancer. 1994;73:1652–9. doi: 10.1002/1097-0142(19940315)73:6<1652::aid-cncr2820730617>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell PW, Griffin AM, Eward WC, et al. The effect of the setting of a positive surgical margin in soft tissue sarcoma. Cancer. 2014;120:2866–75. doi: 10.1002/cncr.28793. [DOI] [PubMed] [Google Scholar]
- 9.Pan E, Goldberg SI, Chen YL, et al. Role of post-operative radiation boost for soft tissue sarcomas with positive margins following pre-operative radiation and surgery. J Surg Oncol. 2014;110:817–22. doi: 10.1002/jso.23741. [DOI] [PubMed] [Google Scholar]
- 10.Al Yami A, Griffin AM, Ferguson PC, et al. Positive surgical margins in soft tissue sarcoma treated with preoperative radiation: is a postoperative boost necessary? Int J Radiat Oncol Biol Phys. 2010;77:1191–7. doi: 10.1016/j.ijrobp.2009.06.074. [DOI] [PubMed] [Google Scholar]
- 11.Dagan R, Indelicato DJ, McGee L, et al. The significance of a marginal excision after preoperative radiation therapy for soft tissue sarcoma of the extremity. Cancer. 2012;118:3199–207. doi: 10.1002/cncr.26489. [DOI] [PubMed] [Google Scholar]
- 12.Dickie CI, Griffin AM, Parent AL, et al. The relationship between local recurrence and radiotherapy treatment volume for soft tissue sarcomas treated with external beam radiotherapy and function preservation surgery. Int J Radiat Oncol Biol Phys. 2012;82:1528–34. doi: 10.1016/j.ijrobp.2011.03.061. [DOI] [PubMed] [Google Scholar]
- 13.Wang D, Zhang Q, Eisenberg BL, et al. Significant Reduction of Late Toxicities in Patients With Extremity Sarcoma Treated With Image-Guided Radiation Therapy to a Reduced Target Volume: Results of Radiation Therapy Oncology Group RTOG-0630 Trial. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.58.5828. JCO.2014.58.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim B, Chen YL, Kirsch DG, et al. An effective preoperative three-dimensional radiotherapy target volume for extremity soft tissue sarcoma and the effect of margin width on local control. Int J Radiat Oncol Biol Phys. 2010;77:843–50. doi: 10.1016/j.ijrobp.2009.06.086. [DOI] [PubMed] [Google Scholar]
- 15.Cleator SJ, Cottrill C, Harmer C. Pattern of local recurrence after conservative surgery and radiotherapy for soft tissue sarcoma. Sarcoma. 2001;5:83–8. doi: 10.1155/S1357714X01000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holloway CL, Delaney TF, Alektiar KM, et al. American Brachytherapy Society (ABS) consensus statement for sarcoma brachytherapy. Brachytherapy. 2013;12:179–90. doi: 10.1016/j.brachy.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 17.von Mehren M, Randall RL, Benjamin RS, et al. National Comprehensive Cancer Network. Soft tissue sarcoma, version 2.2014. J Natl Compr Canc Netw. 2014;12:473–83. doi: 10.6004/jnccn.2014.0053. [DOI] [PubMed] [Google Scholar]
- 18.The ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25:iii102–112. doi: 10.1093/annonc/mdu254. [DOI] [PubMed] [Google Scholar]
- 19.Pisters PW, Pollock RE, Lewis VO, et al. Long-term results of prospective trial of surgery alone with selective use of radiation for patients with T1 extremity and trunk soft tissue sarcomas. Ann Surg. 2007;246:675–81. doi: 10.1097/SLA.0b013e318155a9ae. [DOI] [PubMed] [Google Scholar]
- 20.Baldini EH, Goldberg J, Jenner C, et al. Long-term outcomes after function-sparing surgery without radiotherapy for soft tissue sarcoma of the extremities and trunk. J Clin Oncol. 1999;17:3252–9. doi: 10.1200/JCO.1999.17.10.3252. [DOI] [PubMed] [Google Scholar]
- 21.Gronchi A. Individualizing the use/non-use of radiation therapy (RT) in soft tissue sarcoma (STS): When abstention is better than care. J Surg Oncol. 2015;111:133–4. doi: 10.1002/jso.23827. [DOI] [PubMed] [Google Scholar]
- 22.Cahlon O, Brennan MF, Jia X, et al. A postoperative nomogram for local recurrence risk in extremity soft tissue sarcomas after limb-sparing surgery without adjuvant radiation. Ann Surg. 2012;255:343–7. doi: 10.1097/SLA.0b013e3182367aa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859–868. doi: 10.1200/JCO.1996.14.3.859. [DOI] [PubMed] [Google Scholar]
- 24.Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. doi: 10.1200/JCO.1998.16.1.197. [DOI] [PubMed] [Google Scholar]
- 25.Werkhoven E, Hart G, van Tinteren H, et al. Nomogram to predict ipsilateral breast relapse based on pathology review from the EORTC 22881–10882 boost versus no boost trial. Radiother Oncol. 2011;100:101–7. doi: 10.1016/j.radonc.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Eilber F, Eckhardt J, Rosen G, et al. Preoperative therapy for soft tissue sarcoma. Heamatol Oncol Clin North Am. 1995;9:817–23. [PubMed] [Google Scholar]
- 27.Eilber F, Giuliano A, Huth JH, et al. Neoadjuvant chemotherapy, radiation, and limited surgery for high grade soft tissue sarcoma of the extremity. In: Ryan JR, Baker LO, editors. Recent concepts in sarcoma treatment. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1988. pp. 115–122. [Google Scholar]
- 28.Temple WJ, Temple CL, Arthur K, et al. Prospective cohort study of neoadjuvant treatment in conservative surgery of soft tissue sarcomas. Ann Surg Oncol. 1997;4:586–90. doi: 10.1007/BF02305541. [DOI] [PubMed] [Google Scholar]
- 29.Thames HD, Suit HD. Tumor radioresponsiveness versus fractionation sensitivity. Int J Radiat Oncol Biol Phys. 1986;12:687–91. doi: 10.1016/0360-3016(86)90081-7. [DOI] [PubMed] [Google Scholar]
- 30.Kraybill WG, Harris J, Spiro IJ, et al. Phase II study of neoadjuvant chemotherapy and radiation therapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. J Clin Oncol. 2006;24:619–25. doi: 10.1200/JCO.2005.02.5577. [DOI] [PubMed] [Google Scholar]
- 31.Kosela-Paterczyk H, Szacht M, Morysinski T, et al. Preoperative hypofractionated radiotherapy in the treatment of localized soft tissue sarcomas. Eur J Surg Oncol. 2014;40:1641–7. doi: 10.1016/j.ejso.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 32.Betgen A, Haas RL, Sonke JJ. Volume changes in soft tissue sarcomas during preoperative radiotherapy of extremities evaluated using cone-beam CT. J Radiat Oncol. 2013;2:55–62. doi: 10.1007/s13566-012-0085-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung PW, Deheshi BM, Ferguson PC, et al. Radiosensitivity translates into excellent local control in extremity myxoid liposarcoma: a comparison with other soft tissue sarcomas. Cancer. 2009;115:3254–61. doi: 10.1002/cncr.24375. [DOI] [PubMed] [Google Scholar]
- 34.Guadagnolo BA, Zagars GK, Ballo MT, et al. Excellent local control rates and distinctive patterns of failure in myxoid liposarcoma treated with conservation surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:760–5. doi: 10.1016/j.ijrobp.2007.07.2337. [DOI] [PubMed] [Google Scholar]
- 35.de Vreeze RS, de Jong D, Haas RL, et al. Effectiveness of radiotherapy in myxoid sarcomas is associated with a dense vascular pattern. Int J Radiat Oncol Biol Phys. 2008;72:1480–7. doi: 10.1016/j.ijrobp.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 36.O’Sullivan B, Griffin AM, Dickie CI, et al. Phase 2 study of preoperative image-guided intensity-modulated radiation therapy to reduce wound and combined modality morbidities in lower extremity soft tissue sarcoma. Cancer. 2013;119:1878–84. doi: 10.1002/cncr.27951. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz A, Rebecca A, Smith A, et al. Risk factors for significant wound complications following wide resection of extremity soft tissue sarcomas. Clin Orthop Relat Res. 2013;471:3612–7. doi: 10.1007/s11999-013-3130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nielsen OS, Cummings B, O’Sullivan B, et al. Preoperative and postoperative irradiation of soft tissue sarcomas: effect of radiation field size. Int J Radiat Oncol Biol Phys. 1991;21:1595–9. doi: 10.1016/0360-3016(91)90337-4. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Jacobs MA, Fayad L. Therapeutic response in musculoskeletal soft tissue sarcomas: evaluation by MRI. NMR Biomed. 2011;24:750–63. doi: 10.1002/nbm.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tirumani SH, Jagannathan JP, O’Regan K, et al. Molecular targeted therapies in non-GIST soft tissue sarcomas: what the radiologist needs to know. Cancer Imaging. 2013;13:197–211. doi: 10.1102/1470-7330.2013.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer JM, Perlewitz KS, Hayden JB, et al. Phase I trial of preoperative chemoradiation plus sorafenib for high-risk extremity soft tissue sarcomas with dynamic contrast-enhanced MRI correlates. Clin Cancer Res. 2013;19:6902–11. doi: 10.1158/1078-0432.CCR-13-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canter RJ, Martinez SR, Tamurian RM, et al. Radiographic and histologic response to neoadjuvant radiotherapy in patients with soft tissue sarcoma. Ann Surg Oncol. 2010;17:2578–84. doi: 10.1245/s10434-010-1156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah D, Borys D, Martinez SR, et al. Complete pathologic response to neoadjuvant radiotherapy is predictive of oncological outcome in patients with soft tissue sarcoma. Anticancer Res. 2012;32:3911–5. [PMC free article] [PubMed] [Google Scholar]
- 44.Mullen JT, Hornicek FJ, Harmon DC, et al. Prognostic significance of treatment-induced pathologic necrosis in extremity and truncal soft tissue sarcoma after neoadjuvant chemoradiotherapy. Cancer. 2014;120:3676–82. doi: 10.1002/cncr.28945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeLaney TF, Spiro IJ, Suit HD, et al. Neoadjuvant chemotherapy and radiotherapy for large extremity soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2003;56:1117–27. doi: 10.1016/s0360-3016(03)00186-x. [DOI] [PubMed] [Google Scholar]
- 46.Mullen JT, Kobayashi W, Wang JJ, et al. Long-term follow-up of patients treated with neoadjuvant chemotherapy and radiotherapy for large, extremity soft tissue sarcomas. Cancer. 2012;118:3758–65. doi: 10.1002/cncr.26696. [DOI] [PubMed] [Google Scholar]
- 47.Kraybill WG, Harris J, Spiro IJ, et al. Long-term results of a phase 2 study of neoadjuvant chemotherapy and radiotherapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: Radiation Therapy Oncology Group Trial 9514. Cancer. 2010;116:4613–21. doi: 10.1002/cncr.25350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gronchi A, De Paoli A, Dani C, et al. Preoperative chemo-radiation therapy for localised retroperitoneal sarcoma: a phase I–II study from the Italian Sarcoma Group. Eur J Cancer. 2014;50:784–92. doi: 10.1016/j.ejca.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 49.MacDermed DM, Miller LL, Peabody TD, et al. Primary tumor necrosis predicts distant control in local advanced soft-tissue sarcomas after preoperative concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1147–53. doi: 10.1016/j.ijrobp.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ryan CW, Montag AG, Hosenpud JR, et al. Histologic response of dose-intense chemotherapy with preoperative hypofractionated radiotherapy for patients with high-risk soft tissue sarcomas. Cancer. 2008;112:2432–9. doi: 10.1002/cncr.23478. [DOI] [PubMed] [Google Scholar]
- 51.Anderson P, Aguilera D, Pearson M, Woo S. Outpatient chemotherapy plus radiotherapy in sarcomas: improving cancer control with radiosensitizing agents. Cancer Control. 2008;15:38–46. doi: 10.1177/107327480801500105. [DOI] [PubMed] [Google Scholar]
- 52.Krikelis D, Judson I. Role of chemotherapy in the management of soft tissue sarcomas. Expert Rev Anticancer Ther. 2010;10:249–60. doi: 10.1586/era.09.176. [DOI] [PubMed] [Google Scholar]
- 53.Gronchi A, Frustaci S, Mercuri M, et al. Short, full-dose adjuvant chemotherapy in high risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol. 2012;30:850–6. doi: 10.1200/JCO.2011.37.7218. [DOI] [PubMed] [Google Scholar]
- 54.Woll PJ, Reichardt P, Le Cesne A, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol. 2012;13:1045–54. doi: 10.1016/S1470-2045(12)70346-7. [DOI] [PubMed] [Google Scholar]
- 55.Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871–82. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verweij J, Sleijfer S. Pazopanib, a new therapy for metastatic soft tissue sarcoma. Expert Opin Pharmacother. 2013;14:929–35. doi: 10.1517/14656566.2013.780030. [DOI] [PubMed] [Google Scholar]
- 57.Senan S, Smit EF. Design of clinical trials of radiation combined with antiangiogenic therapy. Oncologist. 2007;12:465–77. doi: 10.1634/theoncologist.12-4-465. [DOI] [PubMed] [Google Scholar]
- 58.Yoon SS, Duda DG, Karl DL, et al. Phase II study of neoadjuvant bevacizumab and radiotherapy for resectable soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 2011;81:1081–90. doi: 10.1016/j.ijrobp.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Canter RJ, Borys D, Olusanya A, et al. Phase I trial of neoadjuvant conformal radiotherapy plus sorafenib for patients with locally advanced soft tissue sarcoma of the extremity. Ann Surg Oncol. 2014;21:1616–23. doi: 10.1245/s10434-014-3543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lewin J, Khamly KK, Young RJ, et al. A phase Ib/II translational study of sunitinib with neoadjuvant radiotherapy in soft-tissue sarcoma. Br J Cancer. 2014;111:2254–61. doi: 10.1038/bjc.2014.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haas RL, Gelderblom H, Sleijfer S, et al. A phase I study on the combination of neoadjuvant radiotherapy plus pazopanib in patients with locally advanced soft tissue sarcoma of the extremities. Acta Oncol. 2015:1–7. doi: 10.3109/0284186X.2015.1037404. Early Online. [DOI] [PubMed] [Google Scholar]
- 62.Ebos JM, Lee CR, Cruz-Munoz W, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–9. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pàez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jakob J, Rauch G, Wenz F, Hohenberger P. Phase I trial of concurrent sunitinib and radiation therapy as preoperative treatment for soft tissue sarcoma. BMJ Open. 2013;3(9):e003626. doi: 10.1136/bmjopen-2013-003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bonvalot S, Le Pechoux C, De Baere Th, et al. Phase I study of NBTXR3 nanoparticles, in patients with advanced soft tissue sarcoma (STS) J Clin Oncol. 2014;32:5s. (suppl;abstr 10563) [Google Scholar]
- 66.Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. WHO Classification of Tumours of Soft Tissue and Bone. IARC; Lyon: 2013. [Google Scholar]
- 67.Pisters PW, Leung DH, Woodruff J, et al. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14:1679–89. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 68.Haglund KE, Raut CP, Nascimento AF, et al. Recurrence patterns and survival for ptients with Intermediate- and high-grade myxofibrosarcoma. Int J Radiat Oncol Biol Phys. 2012;82:361. doi: 10.1016/j.ijrobp.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 69.Eilber FC, Rosen G, Eckardt J, et al. Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol. 2001;19:3203–9. doi: 10.1200/JCO.2001.19.13.3203. [DOI] [PubMed] [Google Scholar]
- 70.Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–94. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 71.Folkert MR, Singer S, Brennan MF, et al. Comparison of local recurrence with conventional and intensity-modulated radiation therapy for primary soft-tissue sarcomas of the extremity. J Clin Oncol. 2014;32:3236–41. doi: 10.1200/JCO.2013.53.9452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zohar S, Teramukai S, Zhou Y. Bayesian design and conduct of phase II single-arm clinical trials with binary outcomes: A tutorial. Contemporary Clinical Trials. 2008;29:608–16. doi: 10.1016/j.cct.2007.11.005. [DOI] [PubMed] [Google Scholar]