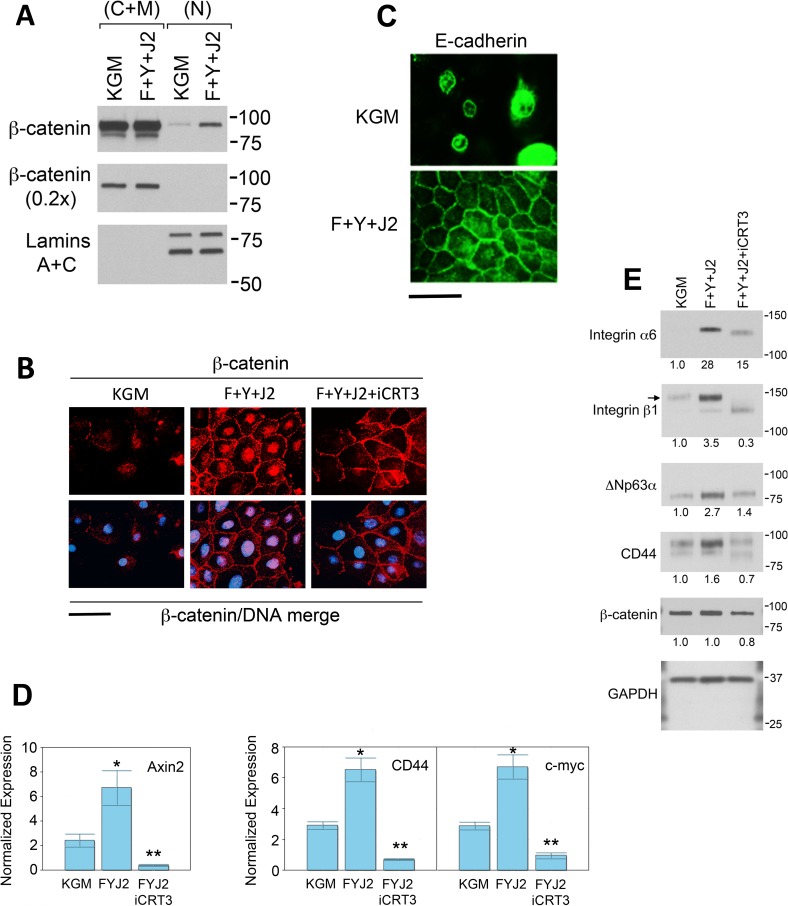
Fig 1. β-catenin-dependent transcription is required for increased expression of epithelial stem cell markers in CR HECs.
(A) Western blots of nuclear (N) and non-nuclear (cytoplasm + membrane; C+M) fractions prepared from HECs maintained in KGM (KGM), or CR conditions (co-cultured with irradiated J2 fibroblasts in F-medium containing 10 μM Y-27632) for 3 d (F+Y+J2). All lanes were derived from equivalent numbers of cells. The lower (0.2x) β-catenin immunoblot was loaded with 1/5 as many cell equivalents as the upper. The efficiency of cell fractionation was verified by distribution of the nuclear scaffolding proteins, lamins A and C. (B) Primary HECs were cultured on coverslips for 3 d in KGM (KGM) or under CR conditions with (F+Y+J2+iCRT3) or without (F+Y+J2) 25 μM iCRT3. The HECs were extracted with TX-100, fixed and labeled with anti-β-catenin antibodies and Hoechst dye as described in Materials and Methods. Scale bar: 10 μm. (C) After 3 d in KGM (KGM) or CR culture (F+Y+J2), HECs on coverslips were fixed without prior detergent extraction and labeled with E-cadherin antibodies to visualize adherins junctions. Scale bar: 10 μm. (D) After 3 d in KGM (KGM), or CR cell culture with (FYJ2iCRT3) or without (FYJ2) 25 μM iCRT3, J2 cells were removed from 25-cm2 flasks by differential trypsin treatment and HECs processed for qRT-PCR analysis to determine levels of β-catenin-dependent mRNA transcripts. Error bars indicate standard deviation (S.D.) from the mean. (*) P-value < .00001 relative to KGM. (**) P-value < .00001 relative to FYJ2. (E) HECs were maintained for 3 d in KGM (KGM), or CR culture with (F+Y+J2+iCRT3) or without (F+Y+J2) 25 μM iCRT3 on 10-cm dishes. J2 cells were removed by EDTA treatment and whole-cell lysates of the HECs prepared for quantification of epithelial stem cell markers on Western blots. Lanes contain equal amounts of protein. Molecular mass markers (in kDa) are shown on the right. Arrow indicates Integrin β1 at 140 kD. The lower band at 120 kD appears to result from non-specific staining.