Figure 7. LC-MS analysis of rose bengal oxidized adduct of dG and the peptide N-Ac-AAAKF.
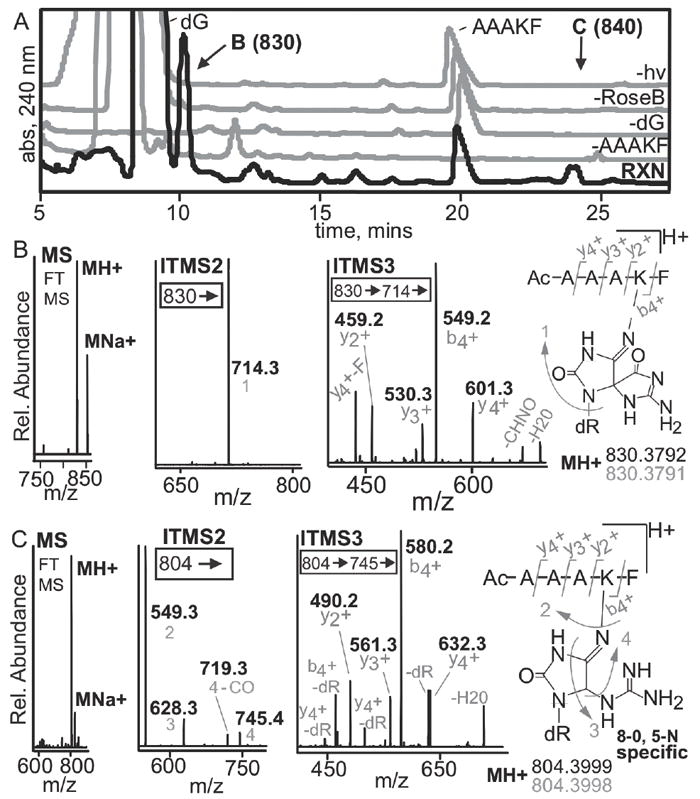
(A) A peptide containing a single crosslink active lysine and 2’-deoxyguanosine were utilized to assess if high-resolution, multi-dimensional MS could be used to establish the location of a crosslink. The HPLC-UV trace of the reaction and the controls are shown. The reaction chromatogram is in black while the controls lacking N-Ac-AAAKF, dG, rose bengal or light are in grey. The 10.5 and 24-minute peaks were unique to the reaction, denoted by B and C respectively. The products were isolated and analyzed by mass spectrometry. (B) Mass spectral analysis of the 10.5-minute peak revealed that it has an observed mass and elemental composition consistent with formation of a spiroiminodihydantoin. MS2 analyses show exclusive fragmentation at guanine. MS3 can be used to determine the site of crosslinking based on the y2+ and b4+ ions. (C) The 24-min peak shows similar results except that more DNA fragmentation is seen in MS2. The 24-min peak is a 5-lys-guanidinohydantoin crosslink based on elemental composition and MS3 fragmentation. All fragments (grey) are acquired using high resolution MS with errors below 1 ppm.
