Abstract
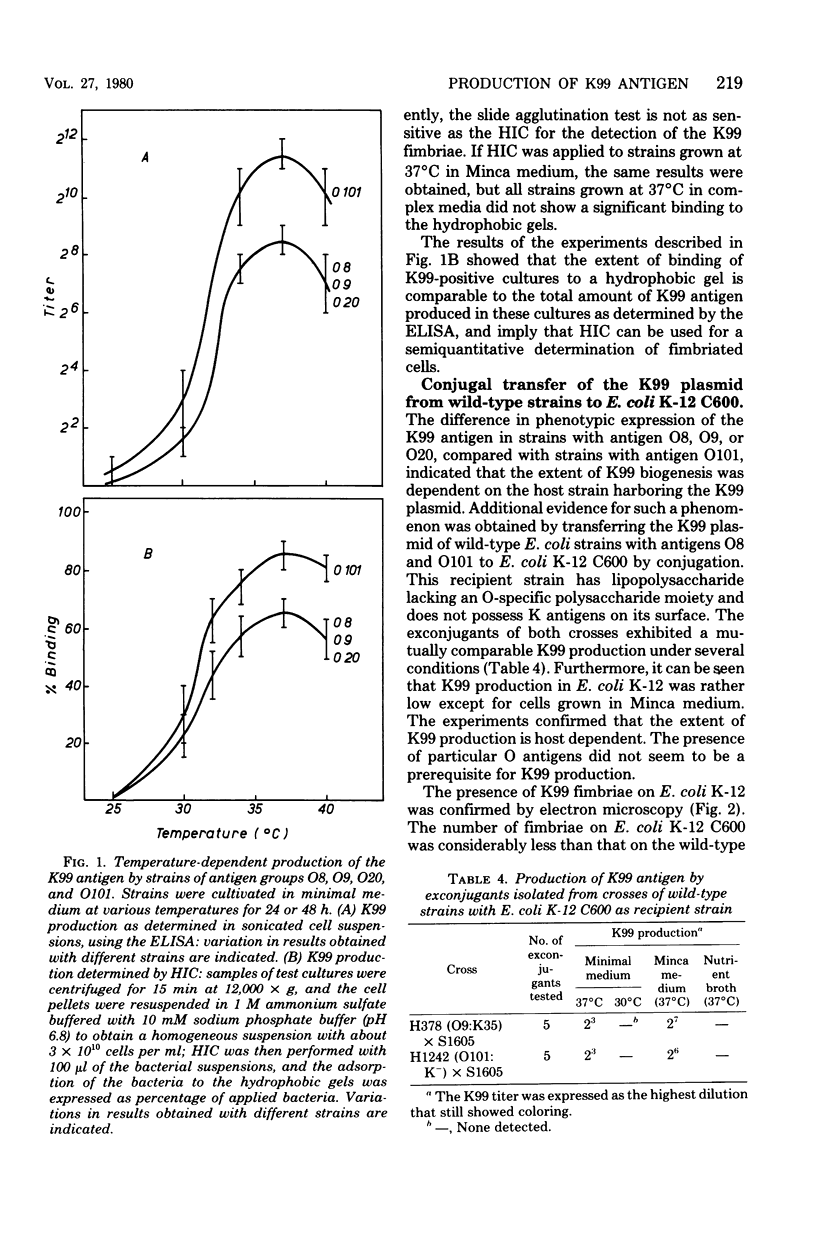
The production of the K99 antigen by enterotoxigenic Escherichia coli strains with various O antigens was investigated by means of slide agglutination tests, enzyme-linked immunosorbent assays, and hydrophobic interaction chromatography. The extent of K99 production appeared to be dependent on the nutrient medium, as well as on the incubation temperature. Minimal salt medium with glucose and semisynthetic Minca medium were the most suitable for K99 production. In complex media the production of K99 antigen was strongly reduced. Optimal amounts of K99 antigen were produced at 37 degrees C. At 30 degrees C, weak production of K99 antigen was detected by hydrophobic interaction chromatography of enzyme-linked immunosorbent assay. Slide agglutination tests were negative with cultures grown below 32 degrees C. The production of K99 antigen appeared to be related to the O antigen carried by the host strain, but it seemed to be independent of the absence or the presence of various K polysaccharide antigens. Under all conditions used, strains with antigen O101 produced about 10 times more K99 antigen than did strains with antigen O8, O9, or O20. Transfer of the K99 plasmid from wild-type strains of different O antigens to E. coliK-12 C600 confirmed that phenotypic expression of the K99 antigen is most probably related to the cell wall composition of the host.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans D. G., Silver R. P., Evans D. J., Jr, Chase D. G., Gorbach S. L. Plasmid-controlled colonization factor associated with virulence in Esherichia coli enterotoxigenic for humans. Infect Immun. 1975 Sep;12(3):656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinée P. A., Jansen W. H., Agterberg C. M. Detection of the K99 antigen by means of agglutination and immunoelectrophoresis in Escherichia coli isolates from calves and its correlation with entertoxigenicity. Infect Immun. 1976 May;13(5):1369–1377. doi: 10.1128/iai.13.5.1369-1377.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinée P. A., Veldkamp J., Jansen W. H. Improved minca medium for the detection of K99 antigen in calf enterotoxigenic strains of Escherichia coli. Infect Immun. 1977 Feb;15(2):676–678. doi: 10.1128/iai.15.2.676-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E. K99 surface antigen of Escherichia coli: purification and partial characterization. Infect Immun. 1977 Jan;15(1):272–279. doi: 10.1128/iai.15.1.272-279.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Nagy B., Moon H. W. Colonization of porcine small intestine by Escherichia coli: colonization and adhesion factors of pig enteropathogens that lack K88. J Infect Dis. 1977 Apr;135(4):531–539. doi: 10.1093/infdis/135.4.531. [DOI] [PubMed] [Google Scholar]
- Mooi F. R., de Graaf F. K., van Embden J. D. Cloning, mapping and expression of the genetic determinant that encodes for the K88ab antigen. Nucleic Acids Res. 1979 Mar;6(3):849–865. doi: 10.1093/nar/6.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Nagy B., Isaacson R. E., Orskov I. Occurrence of K99 antigen on Escherichia coli isolated from pigs and colonization of pig ileum by K99+ enterotoxigenic E. coli from calves and pigs. Infect Immun. 1977 Feb;15(2):614–620. doi: 10.1128/iai.15.2.614-620.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. A., Stevens A. E., Sojka W. J. Preliminary characterization of cell-free K99 antigen isolated from Escherichia coli B41. J Gen Microbiol. 1977 Apr;99(2):353–357. doi: 10.1099/00221287-99-2-353. [DOI] [PubMed] [Google Scholar]
- Myers L. L., Guinée P. A. Occurrence and characteristics of enterotoxigenic Escherichia coli isolated from calves with diarrhea. Infect Immun. 1976 Apr;13(4):1117–1119. doi: 10.1128/iai.13.4.1117-1119.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- ORSKOV I., ORSKOV F., SOJKA W. J., LEACH J. M. Simultaneous occurrence of E. coli B and Lantigens in strains from diseased swine. Influence of cultivation temperature. Two new E. coli Kantigens: K 87 and K 88. Acta Pathol Microbiol Scand. 1961;53:404–422. [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Smith H. W., Sojka W. J. The establishment of K99, a thermolabile, transmissible escherichia coli K antigen, previously called "Kco", possessed by calf and lamb enteropathogenic strains. Acta Pathol Microbiol Scand B. 1975 Feb;83(1):31–36. doi: 10.1111/j.1699-0463.1975.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Further observations on Escherichia coli enterotoxins with particular regard to those produced by atypical piglet strains and by calf and lamb strains: the transmissible nature of these enterotoxins and of a K antigen possessed by calf and lamb strains. J Med Microbiol. 1972 May;5(2):243–250. doi: 10.1099/00222615-5-2-243. [DOI] [PubMed] [Google Scholar]
- Smyth C. J., Jonsson P., Olsson E., Soderlind O., Rosengren J., Hjertén S., Wadström T. Differences in hydrophobic surface characteristics of porcine enteropathogenic Escherichia coli with or without K88 antigen as revealed by hydrophobic interaction chromatography. Infect Immun. 1978 Nov;22(2):462–472. doi: 10.1128/iai.22.2.462-472.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]




