Fig. 1. Study design.
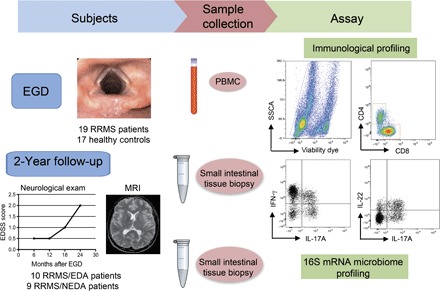
Tissue samples from the duodenal mucosa were collected during EGD from RRMS patients (n = 19) and HCs (n = 17). Simultaneously, we collected 6 to 7 ml of peripheral blood from the same individuals. Total lymphocytes were isolated from one to two fragments of intestinal tissue by collagenase digestion and from peripheral blood by Ficoll gradient. Mucosal immune cells and peripheral blood mononuclear cells (PBMCs) were FACS-analyzed for immunological profiling of the following TH cell subsets: IL-17A+CD4+ TH17 cells, IFN-γ+CD4+ TH1 cells, and IL-22+ TH22 cells. Microbial DNA was extracted from frozen intestinal tissue samples (seven RRMS and seven HC samples), and 16S rRNA sequencing was performed on the Roche 454 platform. At the 2-year end point, we stratified RRMS patients in two cohorts on the basis of evidence of disease activity by clinical and MRI criteria. RRMS patients with no evidence of disease activity in the 2-year follow-up were classified as RRMS/NEDA (n = 9), whereas RRMS patients with evidence of disease activity were classified as RRMS/EDA (n = 10). SSCA, side scatter FACS parameter.
