Table 2. Clinical activity of targeted therapy in BRAF‐mutant metastatic non‐small cell lung cancer.
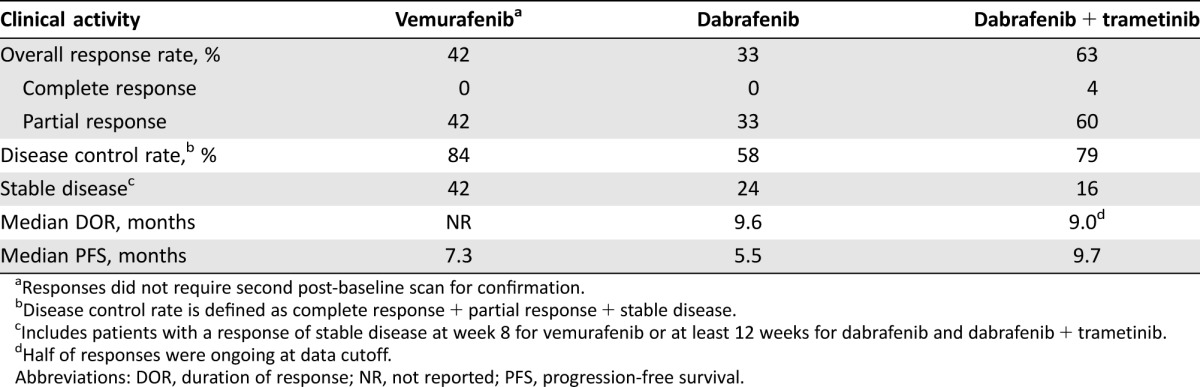
Responses did not require second post‐baseline scan for confirmation.
Disease control rate is defined as complete response + partial response + stable disease.
Includes patients with a response of stable disease at week 8 for vemurafenib or at least 12 weeks for dabrafenib and dabrafenib + trametinib.
Half of responses were ongoing at data cutoff.
Abbreviations: DOR, duration of response; NR, not reported; PFS, progression‐free survival.
