Abstract
Abstract
The Smilax species, widely distributed in tropical region of the world and the warm areas of East Asia and North America, are extensively used as folk medicine to treat inflammatory disorders. Chemical investigation on Smilax species showed they are rich sources of steroidal saponins with diversified structure types, including spirostane, isospirostane, furostane, pregnane, and cholestane. This review mainly summarizes the steroidal saponins (1–104) reported from the genus Smilax between 1967 and 2016, and their biological activities. The relationship between structures of steroidal saponins and related biological activities were briefly discussed.
Graphical Abstract
Keywords: Smilax, Steroidal saponins, Biological activities
Introduction
The genus Smilax (Liliaceae family) comprises about 300 species of climbing shrubs. Plants of the genus are widely distributed in tropical region of the world, and also found in warm areas of East Asia and North America [1]. The juvenile leaves of S. riparia are used as vegetable product. The rhizomes of S. glabra are used in Southeast of China as food supplementary for health. Noteworthily, the rhizomes of Smilax species are most famous for their medical use. The rhizomes of S. china and S. glabra, called “Jin Gang Teng” and “Tu Fu Lin” in Pharmacopoeia of People’s Republic of China respectively, are clinically used to treat chronic pelvic inflammatory disease, rheumatic arthritis and so on [2]. The rhizomes of S. riparia, S. nipponica, S. bockii, S. microphylla, and S. discotis were recorded in the Chinese Herbal Medicines to treat joint pain, edema, and rheumatoid arthritis [3].
Previous studies on chemical constituents of Smilax species have disclosed the presence of steroidal saponins, flavonoids, phenylpropanoids, and stilbenoids [4]. Astilbin, a main flavonoid among Smilax species [5], showed unique immunosuppressive activity, and proved to be the active material basis of Smilax species for the treatment of human immune diseases [6]. Steroidal saponins are characteristic bioactive components of the genus Smilax in terms of chemotaxonomic value and biological activities [7]. So far, 104 steroidal saponins have been reported from 20 different Smilax species. These steroidal saponins showed significant antifungal, cytotoxic, anti-inflammatory, as well as cAMP phosphodiesterase inhibitory activities.
In this review, steroidal saponins reported from the genus Smilax between 1967 and 2016 were listed, and the biological activities of steroidal saponins were also included.
Chemistry of Steroidal Saponins
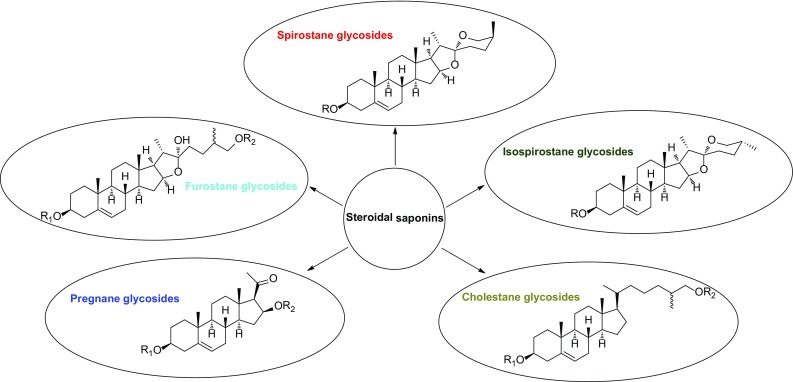
Steroidal saponins from the genus Smilax could be divided into five groups on the basis of the sapogenin structures: spirostane (A), isospirostane (B), furostane (C), pregnane (D), and cholestane (E) (Fig. 1). They are mostly mono- or bisdesmosides. A carbohydrate chain is always attached to the C-3 position of sapogenin by an ether linkage. Additionally, C-26 position of furostane-type saponin is always etherified with a glucopyranosyl moiety. So far only one steroidal saponin from the genus Smilax, (25S)-26-O-β-d-glucopyranosyl-5β-furostan-1β,3β,22α,26-tetraol-1-O-β-d-glucopyranoside (92), has a glucopyranosyl moiety linked to the C-1 position. The sugar residues consist of linear or branched saccharidic chains, made up most often of glucopyranosyl (Glcp), rhamnopyranosyl (Rhap), galactopyranosyl (Galp), fructofuronosyl (Fruf), and arabinopyranosyl (Arap) moieties (Fig. 1).
Fig. 1.
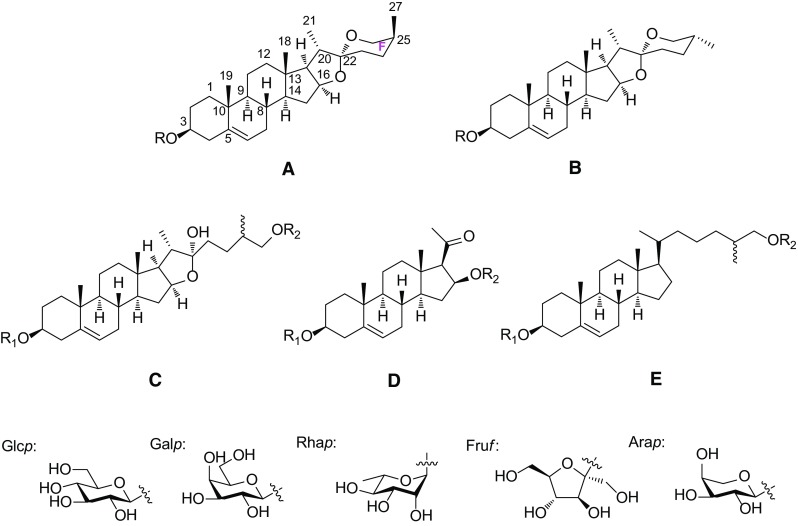
Structures of a a spirostane backbone, b an isospirostane backbone, c a furostane backbone, d a pregnane backbone, e a cholestane backone, a glucopyranosyl moiety (Glcp), a galactopyranosyl moiety (Galp), a rhamnopyranosyl moiety (Rhap), a frutofuranosyl moiety (Furf) and an arabinopyranosyl moiety
Spirostane-Type Saponins 1–11
Spirostane-type saponins are monodesmosidic glycosides with six rings A–F in sapogenin. They are characterized by an axial oriented methyl or hydroxymethyl (C-27) on F ring. The sapogenin of spirostane glycosides 1–11 possess either a cis or a trans fusion between rings A and B, or a double bond between C-5 and C-6, leading to 5α (neotigogenin), 5β (sarsasapogenin), and Δ5 (narthogenin) subtypes (Fig. 2). Neotigogenin glycosides 1–5, and 10 have been isolated from S. riparia [8], S. nipponica [9], and S. officinalis [7]. Both neotigogenin glycosides 5, 10 and sarsasapogenin glycoside 6 were identified from the rhizomes of S. officinalis [7]. Sarsasapogenin glycosides 7–9 were isolated from the root of S. aspera subsp. mauritanica [10], and S. ornata Lem. [11]. Compound 11, with a hydroxyl substitution on C-27, was the only narthogenin glycoside reported from Smilax species so far.
Fig. 2.
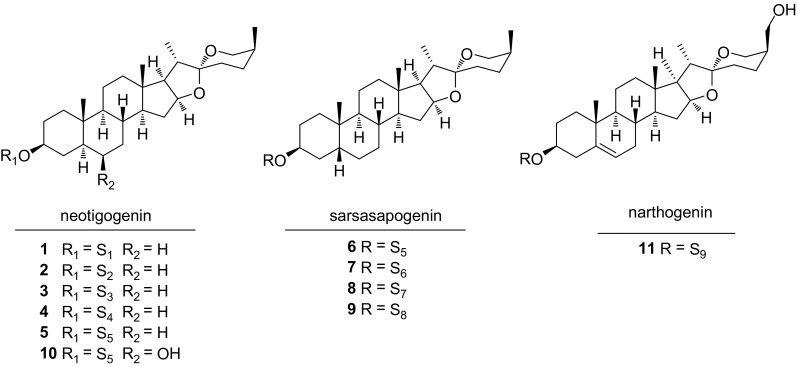
Structures of compounds 1–11
Isospirostane-Type Saponins 12–47
Isospirostane-type saponins are also monodesmosidic glycosides characterized by an equatorial oriented methyl or hydroxymethyl (C-27) on F ring. The isospirostane-type saponins 12–47 could be classified into four subtypes on the basis of sapogenin structures, including diosgenin, laxogenin, tigogenin, and smilagenin (Fig. 3). The variations of these sapogenins mainly comprise dehydrogenation between C-5 and C-6, carbonylation at C-6, hydroxylation at C-17 or C-27, and cis/trans fusion between rings A and B. Diosgenin glycosides 12–30 were characterized by a double bond between C-5 and C-6. Diosgenin-3-O-α-l-rhamnopyranoside (12) was the first diosgenin glycoside reported from the epigeal part of S. excelsa in 1975 [12]. Dioscin (13) was widely distributed among the Smilax species, including S. china [8], S. menispermoidea [13], S. lebrunii [14], S. nigrescens [15], S. stans [16], S. excels [17], S. microphylla [18], and S. bockii [19]. Parisyunnanosides C–E (18–20), with hydroxyl substitutions at C-7 or C-12, were isolated from the stems of S. riparia [20]. The occurrences of parisyunnanoside in the genus Smilax indicated the close chemtaxonomic relationship between the genus Smilax and Paris. Three isonarthogenin glycosides 24, 25, and 28 were isolated from S. scobinicaulis, together with two tigogenin glycosides 38–39 [21]. Sieboldogenin (33), with an additional hydroxyl substitution on C-27 in comparison with laxogenin, was identified from the ethyl acetate fraction of S. china [22]. Laxogenin glycosides 34–36 were founded in S. sieboldii [23]. Parisvietnaside A (37), characterized by a double bond between C-7 and C-8, was obtained from the roots and rhizomes of S. riparia [24]. The smilagenin glycosides 42–47 with a cis fusion rings A and B were isolated from the roots of S. medica [25, 26]. Hydroxyl substitution on C-7 or C-12, and double bond between C-7 and C-8 are the rare cases within the steroidal saponins of the genus Smilax.
Fig. 3.
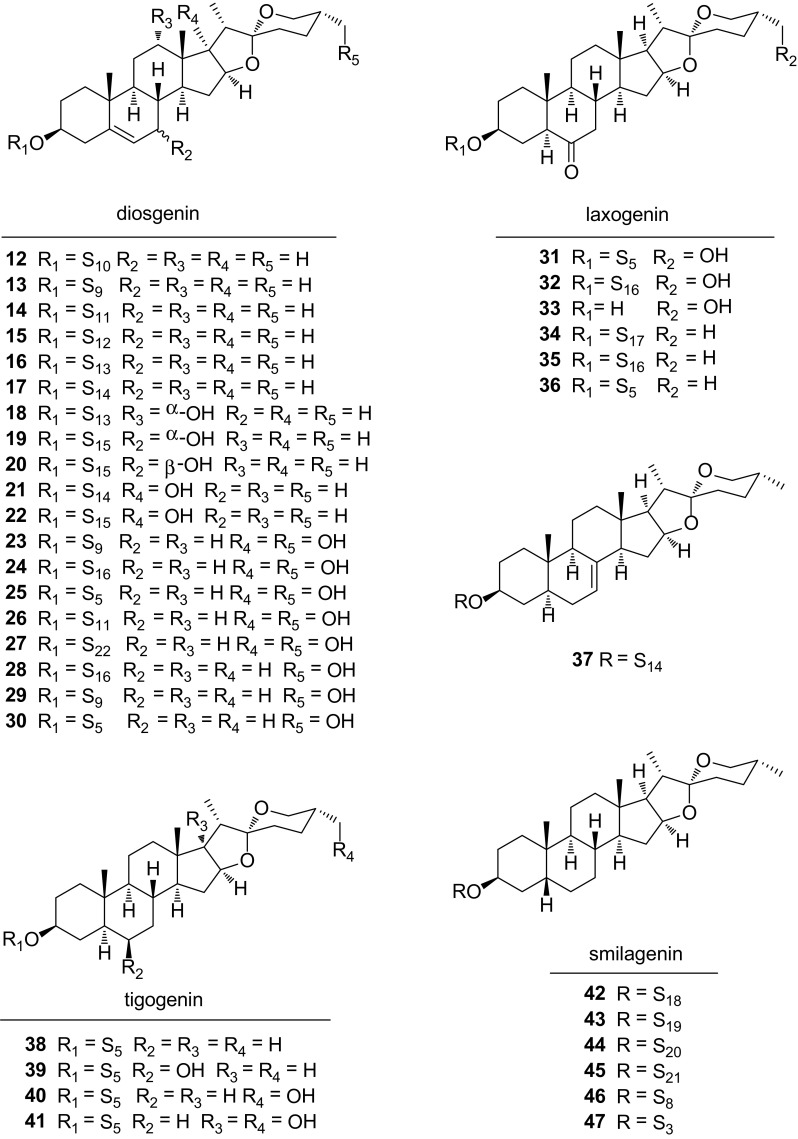
Structures of compounds 12–47
Furostane-Type Saponins 48–93
Furostane-type saponins, F ring opened spirostanol glycosides, are another important group of steroidal saponins within Smilax species. The hemiketal hydroxy attached to the C-22 position of furostanol glycosides were sometimes methylated or dehydrated. The methylated derivatives were generally considered to be artifacts. Furostanol glycosides with both 25R and 25S configurations were reported from the genus Smilax. Additionally, furostanol glycosides always have two sugar chains attached to the C-3 and C-26 positions of the aglycone moiety (Fig. 4). Methylprotodioscin (48), protodioscin (59), and pseudoprotodioscin (60) were common constituents among the different Smilax species (Table 1). Compounds 50, isolated from the roots of S. bockii, increased the nerve growth factor (NGF)-induced neurite outgrowth in PC 12D cells by 49% in comparison with the blank control at the concentration of 60 μg/mL [19]. Compounds 53–55, identified from the rhizomes of S. excelsa, were the only three steroidal saponins with acylated sugars moieties within the genus Smilax [17]. Furostane glycosides 62 and 63, with an oxygenated C-15, were isolated from the tubers of S. china [27]. Interestingly, the spirostane or isospirostane glycosides with an oxygenated C-15 have never been reported from Smilax so far. Compounds 67–70 with carbonylation on C-6 were isolated from the roots and rhizomes of S. scobinicaulis, together with a spirostane glycoside 35, and three furostane glycosides 89–91 [28]. Compounds 76 and 77, isolated from the root of S. officinalis, are the diastereoisomers with opposite configuration at C-5 [29]. Smilaxosides A–C (84, 86, 87), and (25R)-Smilaxchinoside A (85) were obtained tubers from S. china [30]. Of them, compounds 84 and 85 are diastereoisomers with opposite configuration at C-25. Compounds 92 and 93, identified from S. aspera [31], were rare examples with hydroxyl substitution on C-1 within the genus Smilax.
Fig. 4.
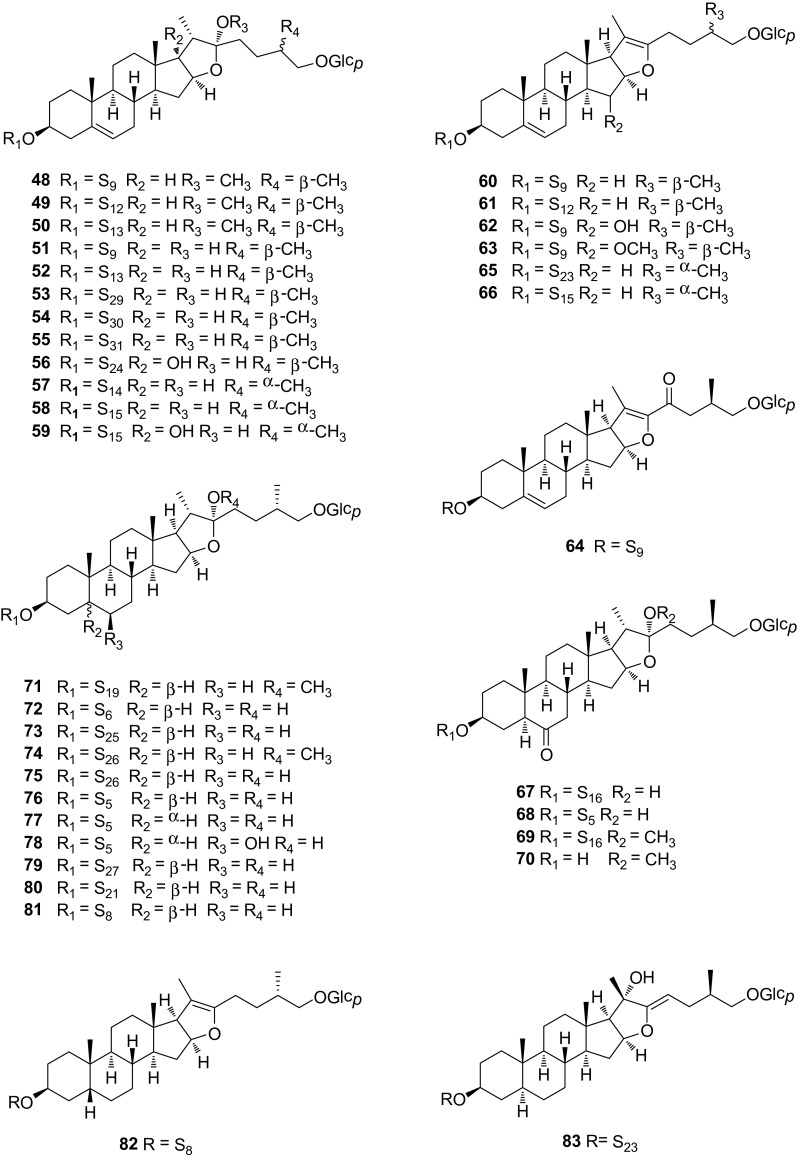
Structures of compounds 48–83
Table 1.
Steroidal saponins from the Genus Smilax
| No. | Name | Plant | Parts | Ref. |
|---|---|---|---|---|
| Spirostane-type saponin | ||||
| 1 | Neotigogenin-3-O-α-l-rhamnopyranosyl-(1 → 6)-β-d-glucopyranoside |
S. riparia
S. lanceaefolia |
Rhizomes and roots Roots |
[8] [37] |
| 2 | Neotigogenin-3-O-β-d-glucopyranosyl-(l → 4)-O-[α-l-rhamnopyranosyl-(l → 6)]-β-d-glucopyranoside | S. riparia | Rhizomes and roots | [8] |
| 3 | Neotigogenin-3-O-β-d-glucopyranoside | S. nipponica | Subterranean | [9] |
| 4 | Smilanippin A | S. nipponica | Subterranean | [9] |
| 5 | Neotigogenin-3-O-β-d-glucopyranosyl-(1 → 4)-O-[α-l-arabinopyranosyl-(1 → 6)]-β-d-glucopyranoside | S. officinalis | Rhizomes | [7] |
| 6 | Sarsasapogenin-3-O-β-d-glucopyranosyl-(1 → 4)-[α-l-arabinopyranosyl- (1 → 6)]-β-d-glucopyranoside | S. officinalis | Rhizomes | [7] |
| 7 | (25S)-5β-Spirostane-3β-ol-3-O-α-l-rhamnopyranosyl-(1 → 2)-β-d-glucopyranosyl-(1 → 2)-β-d-glucopyranoside | S. aspera subsp. mauritanica | Roots | [10] |
| 8 | Curillin G | S. aspera subsp. mauritanica | Roots | [10] |
| 9 | Parillin |
S. aristolochiifolia
S. ornate |
Rhizomes and roots | [38] [11] |
| 10 | (25S)-Spirostan-6β-ol-3-O-β-d-glucopyranosyl-(1 → 4)-O-[α-l-arabinopyranosyl-(1 → 6)]-β-d-glucopyranoside | S. officinalis | Rhizomes | [7] |
| 11 | Trinervuloside C | S. trinervula | Rhizomes and roots | [32] |
| Isospirostane-type saponin | ||||
| 12 | Diosgenin-3-O-α-l-rhamnopyranoside | S. excels | Epigeal part | [12] |
| 13 | Dioscin |
S. china
S. menispermoides S. lebrunii S. nigrescens S. stans S. bockii S. excelsa S. microphylla S. china |
Roots Rhizomes Roots Roots Roots Roots Rhizomes Tubers Tubers |
[8] [13] [14] [15] [16] [19] [17] [18] [30] |
| 14 | Diosgenin-3-O-[α-l-rhamnopyranosyl-(l → 4)]-β-d-glucopyranoside |
S. nigrescens
S. menispermoides S. menispermoides S. china |
Roots Roots Rhizomes Tubers |
[15] [39] [33] [30] |
| 15 | Diosgenin-3-O-[α-l-rhamnopyranosyl-(l → 2)]-β-d-glucopyranoside |
S. nigrescens
S. menispermoides S. microphylla |
Roots Rhizomes Tubers |
[15] [33] [18] |
| 16 | (25R)-Spirostan-5-en-3-O-α-l-rhamnopyranosyl-(l → 2)-[α-l-rhamnopyranosyl-(l → 4)-α-l-rhamnopyranosyl-(l → 4)]-O-β-d-glucopyranoside | S. china | Tubers | [30] |
| 17 | Gracillin | S. microphylla | Tubers | [18] |
| 18 | Parisyunnanoside C | S. riparia | Rhizomes and roots | [20] |
| 19 | Parisyunnanoside D | S. riparia | Rhizomes and roots | [20] |
| 20 | Parisyunnanoside E | S. riparia | Rhizomes and roots | [20] |
| 21 | Paris D | S. riparia | Rhizomes and roots | [20] |
| 22 | Paris H | S. riparia | Rhizomes and roots | [20] |
| 23 | (25R)-Spirost-5-en-3β,17α,27-triol-3-O-α-l-rhamnopyranosyl-(l → 2)-[α-l-rhamnopyranosyl-(l → 4)]-β-d-glucopyranoside | S. menispermoides | Rhizomes | [40] |
| 24 | (25S)-Spirostan-5-en-3β,17α,27-triol-3-O-α-l-arabinopyranosyl-(1 → 6)-β-d-glucopyranoside |
S. lebrunii
S. lebrunii S. scobinicaulis |
Roots Roots Rhizomes and roots |
[14] [41] [21] |
| 25 | (25S)-Spirostan-5-en-3β,17α,27-triol-3-O-β-d-glucopyranosyl-(1 → 4)-[α-l-arabinopyranosyl-(1 → 6)]-β-d-glucopyranoside |
S. lebrunii
S. lebrunii S. scobinicaulis S. scobinicaulis |
Rhizomes Rhizomes Rhizomes and roots Rhizomes |
[33] [42] [21] [43] |
| 26 | (25S)-spirost-5-ene-3β,17α,27-triol-3-O-α-l-rhamnopyranosyl-(l → 4)-β-d-glucopyranoside |
S. menispermoides
S. menispermoides |
Roots Rhizomes |
[39] [33] |
| 27 | (25S)-Spirost-5-en-3β,17α,27-triol-3-O-β-d-galactopyranoside | S. menispermoides | Rhizomes | [33] |
| 28 | (25S)-Spirostan-5-en-3β,27-diol-3-O-α-l-arabinopyranosyl-(1 → 6)-β-d- glucopyranoside |
S. scobinicaulis
S. lebrunii |
Rhizomes and roots Roots |
[21] [14] |
| 29 | Isonarthogenin3-O-α-l-rhamnopyranosyl-(l → 2)-O-[α-l-rhamnopyranosyl-(l → 4)]-β-d-glucopyranoside |
S. china
S. china |
Roots Tubers |
[8] [30] |
| 30 | Smilscobinoside A | S. scobinicaulis | Rhizomes and roots | [44] |
| 31 | Sieboldiin A |
S. sieboldii
S. sieboldii S. scobinicaulis |
Subterranean Rhizomes Rhizomes |
[45] [23] [46] |
| 32 | Sieboldiin B |
S. sieboldii
S. sieboldii S. scobinicaulis S. scobinicaulis |
Subterranean Rhizomes Rhizomes Rhizomes and roots |
[45] [23] [46] [28] |
| 33 | Sieboldogenin | S. china | Rhizomes | [22] |
| 34 | (25R)-5α-Spirostan-3β-ol-6-one-3-O-[α-l-arabinopyranosyl-(l → 4)]-β-d-glucopyranoside |
S. lebrunii
S. lebrunii |
Roots Roots |
[14] [41] |
| 35 | Smilaxin A |
S. sieboldii
S. lebrunii S. scobincaulis |
Subterranean Rhizomes Rhizomes |
[45] [47] [48] |
| 36 | Smilaxin B |
S. sieboldii
S. lebrunii S. sieboldii S. scobincaulis |
Subterranean Rhizomes Rhizomes Rhizomes |
[45] [47] [23] [48] |
| 37 | Parisvietnaside A | S. riparia | Rhizomes and roots | [20] |
| 38 | Smilaxin C |
S. sieboldii
S. sieboldii S. scobinicaulis |
Subterranean Rhizomes Rhizomes and roots |
[45] [23] [21] |
| 39 | (25R)-5α-Spirostan-3β,6β-diol-3-O-β-d-glucopyranosyl-(1 → 4)-[α-l-arabinopyranosyl-(1 → 6)]-β-d-glucopyranoside | S. scobinicaulis | Rhizomes and roots | [21] |
| 40 | Smilscobinoside B | S. scobinicaulis | Rhizomes and roots | [44] |
| 41 | (25R)-5α-Spirostan-3β,17α,27-triol-3-O-β-d-glucopyranosyl-(1 → 4)-[α-l-arabinopyranosyl-(1 → 6)]-β-d-glucopyranosie |
S. scobinicaulis
S. scobinicaulis |
Rhizomes Rhizomes and roots |
[43] [44] |
| 42 | (25R)-5βSpirostan-3β-ol-3-O-β-d-glucopyranosyl-(1 → 6)-[β-d-glucopyranosyl-(1 → 4)]-β-d-glucopyranoside | S. medica | Rhizomes | [25] |
| 43 | (25R)-5β-Spirostan-3β-ol-3-O-β-d-glucopyranosyl-(1 → 6)-[β-d-glucopyranosyl-(1 → 2)]-[β-d-glucopyranosyl-(1 → 4)]-β-d-glucopyranoside | S. medica | Rhizomes | [25] |
| 44 | Disporoside A | S. medica | Rhizomes | [25] |
| 45 | (25R)-5β-Spirostan-3β-ol-3-O-β-d-glucopyranosyl-(1 → 6)-β-d-glucopyranoside | S. medica | Rhizomes | [26] |
| 46 | (25R)-5β-Spirostan-3β-ol-3-O-β-d-glucopyranosyl-(1 → 6)-[β-d-glucopyranosyl-(1 → 2)]-[α-l-rhamnopyranosyl-(l → 4)]-β-d-glucopyranoside | S. medica | Rhizomes | [26] |
| 47 | Smilagenin 3-O-β-d-glucopyranoside | S. medica | Rhizomes | [26] |
| Furostane-type saponin | ||||
| 48 | Methylprotodioscin |
S. china
S. menispermoides S. stans S. bockii S. microphylla S. china S. nigrescens |
Roots Rhizomes Roots Roots Tubers Tubers Roots |
[8] [13] [16] [19] [18] [30] [49] |
| 49 | 26-O-β-d-Glucopyranosyl-(25R)-furostan-5-en-3β,26-diol-22-methoxy-3-O-α-l-rhamnopyranosyl-(l → 2)-β-d-glucopyranoside | S. nigrescens | Roots | [49] |
| 50 | 26-O-β-d-Glucopyranosyl-22α-O-methyl-(25R)-furost-5-en-3β,26-diol-3-O-α-l-rhamnopyranosyl-(l → 4)-α-l-rhamnopyranosyl-(l → 4)-[α-l-rhamnopyranosyl-(l → 2)]-β-d-glucopyranoside | S. bockii | Roots | [19] |
| 51 | Protodioscin |
S. excelsa
S. microphylla S. china |
Rhizomes Tubers Tubers |
[17] [18] [30] |
| 52 | Protodiosgenin-3-O-α-l-rhamnopyranosyl-(1 → 4)-α-l-rhamnopyranosyl(1 → 4)-[α-l-rhamnopyranosyl(1 → 2)]-β-d-glucopyranoside | S. krausiana | Rhizomes | [50] |
| 53 | 26-O-β-d-Glucopyranosyl-22α-hydroxy-(25R)-furost-5-en-3β,26-diol-3-O-[4-O-acetyl-α-l-rhamnopyranosyl]-(l → 2)-[α-l-rhamnopyranosyl-(l → 4)]-β-d-glucopyranoside | S. excelsa | Rhizomes | [17] |
| 54 | 26-O-β-d-Glucopyranosyl-22α-hydroxy-(25R)-furost-5-en-3β,26-diol-3-O-[2-O-acetyl-α-l-rhamnopyranosyl]-(l → 2)-[α-l-rhamnopyranosyl-(l → 4)]-β-d-glucopyranoside | S. excelsa | Rhizomes | [17] |
| 55 | 26-O-β-d-Glucopyranosyl-22α-hydroxy-(25R)-furost-5-en-3β,26-diol-3-O-[3-O-acetyl-α-l-rhamnopyranosyl]-(l → 2)-[α-l-rhamnopyranosyl-(l → 4)]-β-d-glucopyranoside | S. excelsa | Rhizomes | [17] |
| 56 | 26-O-β-d-Glucopyranosyl-(25R)-furostan-5-en-3β,17α-diol-3-O-α-l-rhamnopyranosyl-(l → 2)-α-l-rhamnopyranoside | S. scobiniculis | Rhizomes | [51] |
| 57 | Protogracillin | S. riparia | Rhizomes and roots | [20] |
| 58 | Parisaponin I | S. riparia | Rhizomes and roots | [20] |
| 59 | Parisyunnanoside A | S. riparia | Rhizomes and roots | [20] |
| 60 | Pseudoprotodioscin |
S. china
S. trinervula S. menispermoides S. stans S. excelsa S. china S. nigrescens |
Roots Rhizomes and roots Rhizomes Roots Rhizomes Tubers Roots |
[8] [32] [13] [16] [17] [30] [49] |
| 61 | 26-O-β-d-Glucopyranosyl-(25R)-furostan-5,20(22)-dien-3β,26-diol-3-O-α-l-rhamnopyranosyl-(l → 2)-β-d-glucopyranoside | S. nigrescens | Roots | [49] |
| 62 | 15-Hydroxypseudoprotodioscin | S. china | Tubers | [27] |
| 63 | 15-Methoxypseudoprotodioscin | S. china | Tubers | [27] |
| 64 | 23-Oxopseudoprotodioscin | S. microphylla | Tubers | [18] |
| 65 | 26-O-β-d-Glucopyranosyl-(25S)-5-furosa-20(22)-en-3β,26-diol-3-O-α-l-rhamnopyranosyl-(l → 2)-O-[α-l-rhamnopyranosyl-(l → 6)]-β-d-glucopyranoside | S. riparia | Roots | [52] |
| 66 | Pseudoproto-pb | S. riparia | Rhizomes and roots | [20] |
| 67 | 26-O-β-d-Glucopyranosyl-(25R)-5α-furostan-3β,22,26-triol-6-one-3-O-α-l-arabinopyranosyl-(1 → 6)-β-d-glucopyranoside |
S. sieboldii
S. scobinicaulis |
Rhizomes Rhizomes and roots |
[23] [28] |
| 68 | 26-O-β-d-Glucopyranosyl-(25R)-5α-furostan-3β,22,26-triol-6-one-3-O-β-d-glucopyranosyl-(1 → 4)-[α-l-arabinopyranosyl-(1 → 6)]-β-d-glucopyranoside | S. sieboldii | Rhizomes | [23] |
| 69 | 26-O-β-d-Glucopyranosyl-(25R)-5α-furostan-3β,26-diol-22-methoxyl-6-one-3-O-α-l-arabinopyranosyl-(1 → 6)-β-d-glucopyranoside | S. scobinicaulis | Rhizomes and roots | [28] |
| 70 | 26-O-β-d-Glucopyranosyl-(25R)-5α-furostan-3β,26-diol-22-methoxyl-6-one | S. scobinicaulis | Rhizomes and roots | [28] |
| 71 | 26-O-β-d-Glucopyranosyl-(25S)-5β-furostan-3β,26-diol-22α-methoxy-3-O-β-d-glucopyranosyl-(1 → 6)-[β-d-glucopyranosyl-(1 → 2)]-[β-d-glucopyranosyl-(1 → 4)]-β-d-glucopyranoside | S. medica | Rhizomes | [25] |
| 72 | (25S)-26-O-β-d-glucopyranosyl-3β,5β,22α-furostan-3,22,26-triol-3-O-α-l-rhamnopyranosyl-(l → 2)-O-β-d-glucopyranosyl-(l → 2)-O-β-d-glucopyranoside | S. aspera subsp. mauritanica | Roots | [10] |
| 73 | Asparagoside E | S. aspera subsp. mauritanica | Roots | [10] |
| 74 | Asparoside A | S. aspera subsp. mauritanica | Roots | [10] |
| 75 | Asparoside B | S. aspera subsp. mauritanica | Roots | [10] |
| 76 | 26-O-β-d-Glucopyranosyl-(25S)-5β-furostan-3β,22α-diol-3-O-α-l-arabinopyranosyl-(l → 6)-[β-d-glucopyranosyl-(1 → 4)]-β-d-glucopyranoside | S. officinalis | Roots | [29] |
| 77 | 26-O-β-d-Glucopyranosyl-(25S)-5α-furostan-3β,22α-diol-3-O-α-l-arabinopyranosyl-(l → 6)-[β-d-glucopyranosyl-(1 → 4)]-β-d-glucopyranoside | S. officinalis | Roots | [29] |
| 78 | 26-O-β-d-Glucopyranosyl-(25S)-5α-furostan-3β,6β,22α-tetraol-3-O-α-l-arabinopyranosyl-(l → 6)-[β-d-glucopyranosyl-(1 → 4)]-β-d-glucopyrano side | S. officinalis | Roots | [29] |
| 79 | Sarsaparilloside B | S. ornate | Rhizomes and roots | [11] |
| 80 | Sarsaparilloside C | S. ornate | Rhizomes and roots | [11] |
| 81 | Sarsaparilloside | S. ornate | Rhizomes and roots | [11] |
| 82 | Δ20(22)-Sarsaparilloside | S. ornate | Rhizomes and roots | [11] |
| 83 | Riparoside A | S. riparia | Rhizomes and roots | [53] |
| 84 | Smilaxchinoside A | S. china | Tubers | [30] |
| 85 | (25R)-Smilaxchinoside A |
S. china
S. riparia S. riparia S. trinervula |
Tubers Rhizomes and roots Roots Rhizomes and roots |
[30] [20] [54] [32] |
| 86 | Smilaxchinoside B | S. china | Tubers | [30] |
| 87 | Smilaxchinoside C |
S. china
S. riparia |
Tubers Rhizomes and roots |
[30] [20] |
| 88 | Dioscoreside E | S. trinervula | Rhizomes and roots | [32] |
| 89 | (25R)-5α-Furostan-3β,26-diol-20(22)-en-6-one-26-O-β-d-glucopyranoside | S. scobinicaulis | Rhizomes and roots | [28] |
| 90 | (23R,25R)-5α-Furostan-3β,23,26-triol-20(22)-en-6-one-26-O-β-d-glucopyranoside | S. scobinicaulis | Rhizomes and roots | [28] |
| 91 | 26-O-β-d-Glucopyranosyl-(25R)-5α-furostan-3β,26-diol-20(22)-en-6-one-3-O-α-l-arabinopyranosyl-(1 → 6)-β-d-glucopyranoside | S. scobinicaulis | Rhizomes and roots | [28] |
| 92 | (25S)-5β-Furostan-1β,2β,3β,5β,22α,26-hexaol-26-O-β-d-glucopyrano side | S. aspera | Rhizomes | [31] |
| 93 | 26-O-β-d-Glucopyranosyl-(25S)-5β-furostan-1β,3β,22α,26-tetraol-1-O-β-d-glucopyranoside | S. aspera | Rhizomes | [31] |
| Pregane-type saponin | ||||
| 94 | Trinervuloside A | S. trinervula | Rhizomes and roots | [32] |
| 95 | Riparoside B |
S. riparia
S. riparia S. riparia |
Rhizomes and roots Rhizomes and roots Rhizomes and roots |
[20] [55] [53] |
| 96 | Timosaponin J |
S.riparia
S. riparia |
Rhizomes and roots Rhizomes and roots |
[20] [55] |
| 97 | Timosaponin K | S. riparia | Rhizomes and roots | [20] |
| 98 | Trinervuloside B | S. trinervula | Rhizomes and roots | [32] |
| 99 | Pregna-5,16-diene-3β-ol-20-one-3-O-α-l-rhamnopyranosyl-(l → 2)-O-[α-l-rhamnopyranosyl-(l → 4)]-β-d-glucopyranoside |
S. nigrescens
S. bockii S. menispermoides |
Roots Roots Rhizomes |
[15] [19] [33] |
| 100 | Pregna-5,16-diene-3β-ol-20-one-3-O-α-l-rhamnopyranosyl-(l → 4)-α-l-rhamnopyranosyl-(l → 4)-[α-l-rhamnopyranosyl-(l → 2)]-β-d-glucopyranoside | S. bockii | Roots | [19] |
| 101 | Pregna-5,16-diene-3β-ol-20-one-3-O-α-l-rhamnopyranosyl-(l → 2)-[α-l-rhamnopyranosyl-(l → 4)]-α-l-rhamnopyranosyl-(l → 2)-β-d-glucopyranoside | S. microphylla | Tubers | [18] |
| 102 | Pallidfloside D | S. riparia | Rhizomes and roots | [20] |
| Cholestane-type saponin | ||||
| 103 | Anguivioside XV | S. trinervula | Rhizomes and roots | [32] |
| 104 | Smilaxchinoside D | S. china | Tubers | [30] |
Pregnane-Type Saponins 94–102 and Others 103–104
Pregane-type saponins are C21 steroidal saponins with a sugar moiety linked to the alcoholic hydroxyl group of the sapogenin, most frequently at C-3. Compounds 94–98 are not real pregnane-type saponins from the perspective of biosynthetic pathway. Possibly, they are biosynthetically formed through oxidative cleavage of the double bond between C-20 and C-22 in furostane structures. Compounds 94 and 98 were isolated from the rhizomes and roots of S. trinervula, together with compounds 11, 60, 85, 88, and 103 [32]. Pregnane glycosides 99–102 were found in S. nigrescens [15], S. menispermoidea [33], S. bockii [19], S. microphylla [18], and S. riparia [20]. Compounds 103 and 104, isolated from S. trinervula and S. china respectively, are belonged to cholestane-type saponins, or open chain saponins in another way of saying [34]. S. riparia saponins, from which compounds 18–22, 57–59, 66, 85, 87, 95–97, and 102 were identified, exhibited the synergistic effects with allopurinolin in reducing serum uric acid levels and increasing the urine uric acid level in a hyperuricemic mouse model [20]. The attenuation of hyperuricemia-induced renal dysfunction was linked to the inhibition of serum and hepatic xanthine oxidase, the down-regulation of renal mURAT1 and GLUT9, and the up-regulation of mOAT1. Structures of steroidal saponins (94–104) are shown in Fig. 5.
Fig. 5.
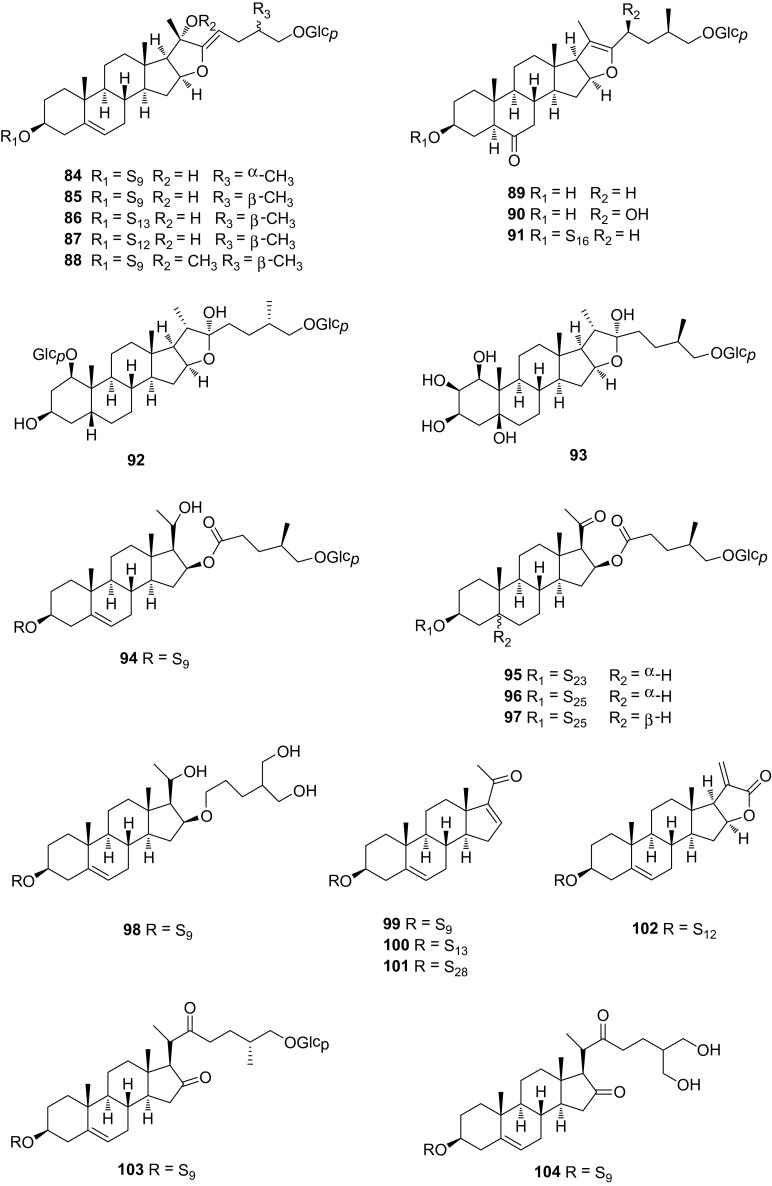
Structures of compounds 84–104
Biological Activities of Steroidal Saponins
Steroidal saponins are considered to be responsible for pharmacological properties of Smilax species. Many pharmacological in vitro and in vivo studies revealed significant biological activities, including cAMP phosphodiesterase inhibitory, anti-fungal, cytotoxic, and anti-inflammatory activities.
cAMP Phosphodiesterase Inhibitory Activity
The cAMP phosphodiesterase is an enzyme that degrades the phosphodiester bond in the second messenger molecule cAMP. It regulates the localization, duration, and amplitude of cyclic nucleotide signaling within subcellular domains. Compounds 1, 2, 29 and 60, showed cAMP phosphodiesterase inhibitory activities with IC50 values of 102, 55, 93, and 47 μM, respectively, which were almost equal to that of positive control papaverine (IC50 = 30 μM) [8]. Laxogenin glycosides 34, 35, and isospirostanol glycoside 38 displayed cAMP phosphodiesterase inhibitory activities with IC50 values of 83, 34, and 32 μM, respectively. While compound 36, with an additional hydroxyl substitution on C-27 in comparison with compound 34, showed no obvious inhibitory activity. Furostane glycosides 67–68 were inactive [23].
Antifungal Activity
C27 steroidal glycosides are well known for their antifungal activities [35]. Sarsasapogenin glycosides 7, 8, and four furostane glycosides 72–75, were tested for their antifungal activity. Compound 8 showed antifungal activity against three human pathogenic species, Candida albicans, C. glabrata, and C. tropicalis, with minimal inhibitory concentration (MIC) values of 25, 25 and 50 μg/mL, respectively. While compounds 7 and 72–75 showed no obvious antifungal activity at concentration of 200 μg/mL [10]. Six smilagenin glycosides 42–47 and a furostane glycoside 71 were also evaluated for their antifungal activities against these three pathogenic species. Compounds 42–46 demonstrated moderate antifungal activity with MIC values between 12.5 and 50 μg/mL [25, 26]. With regard to structure–activity relationships between the saponin structures and antifungal activities, the following points were suggested: (1) the close F ring is essential for the anti-fungal activities. (2) The cis/trans fusion between rings A and B has no significant difference in terms of antifungal activities. (3) Steroidal saponins bearing a saccharidic chain with more than one sugar were better antifungal agents (Figs. 6, 7).
Fig. 6.
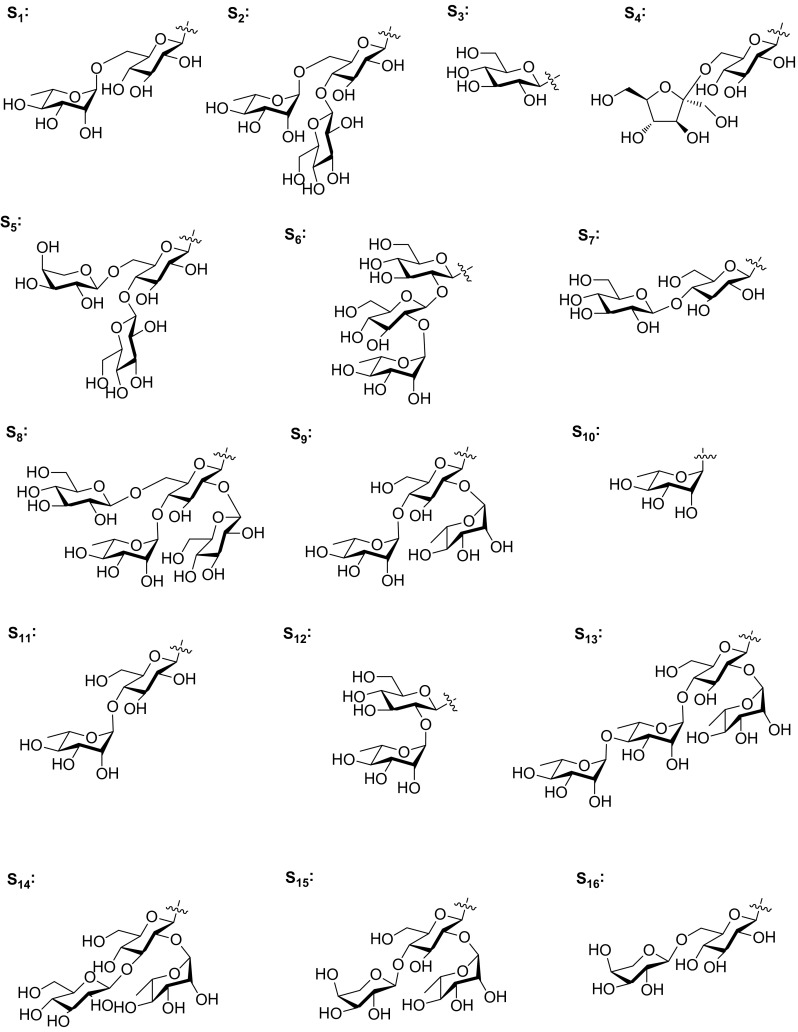
Sugar residues of S1–S16
Fig. 7.
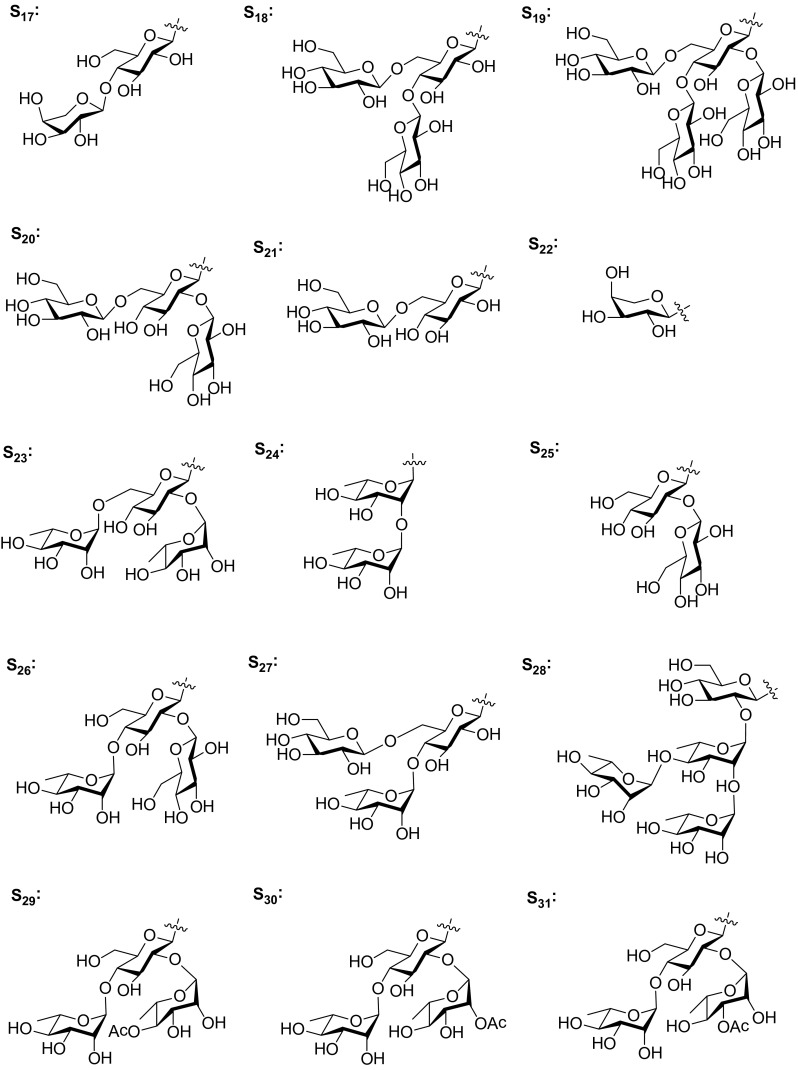
Sugar residues of S17–S31
Cytotoxicity
Spirostane glycoside 9 and four furostane glycosides 79–82 were evaluated for their cytotoxicities against six human cancer cells (NFF, Hela, HT29, MCF7, MM96L, and K562). Compounds 79 and 80 selectively inhibited the proliferation of the HT29 colon cancer cell lines with IC50 values of 4.8 and 5.0 μg/mL, respectively; while compounds 80 and 81 showed significant cytotoxicities aganist MCF7 cell lines with IC50 values of 9.5 and 3.4 μg/mL, respectively [11]. Compounds 24, 25, 28, 38, and 39, were evaluated for the cytotoxicities against three human cancer cell lines (A549, LAC and Hela). Only compound 38 possessed significant cytotoxicities with IC50 values of 3.70, 5.70 and 3.64 μM, respectively [21]. Another cytotoxic compound is isospirostane glycoside 32, which displayed potent cytotoxicities against the Hela and SMMC-7221 cancer cell lines with IC50 values of 9.73 ± 1.64 and 21.54 ± 1.64 μM, respectively [28]. The above results indicated that the hydroxyl substitutions on C-6 or C-17 of isospirostane glycosides decrease the cytotoxicities. Furostane glycoside 69 showed cytotoxicities against the Hela and SMMC-7221 cancer cell lines with IC50 values of 18.79 ± 1.12 and 28.57 ± 1.57 μM, respectively; while the demethylated analogue 67 and the dehydrated analogues 89–91 showed no obvious cytotoxicities. Additionally, the sapogenin 70 was less cytotoxicities than that of corresponding glycoside 69 [28]. Compounds 11, 60, 85, 88, 94, 98 and 103, were tested for their cytotoxicities against SHSY5Y, SGC-7901, HCT-116 and Lovo cell lines. Only compound 98 showed significant cytotoxicities against SGC-7901 and HCT-116 cell lines with IC50 values of 8.1 and 5.5 μM, respectively [32].
Anti-inflammatory Activity
The aqueous extracts of the tubers of S. china showed the similar anti-inflammatory effects in vivo to that of acetylsalicylic acid (200 mg/kg, i.g.) [36]. Sieboldogenin (33) showed significant lipoxygenase inhibition activity with IC50 value of 38 μM. It also exhibited significant inhibition on carrageenan-induced hind paw oedema at the doses of 10 and 50 mg/kg [22]. Compounds 13, 14, 16, 48, 84–87, and 104 inhibited the lipoposaccharide (LPS) induced prostaglandin E2 (PGE2) production in murine peritoneal macrophages by 81.5, 81.7, 76.5, 82.5, 76.1, 59.1, 78.5, 75.9, and 82.0%, respectively, at the concentration of 10 μM. These nine compounds also moderately inhibited the tumor necrosis factor α (TNFα) production on LPS stimulated murine peritoneal macrophages [30].
Prospects
The plants of the genus Smilax are widely spread in China. Their medical use for the treatment of inflammation and rheumatism has a long history in folk China. Previous studies on chemical constituents of Smilax sp. yielded diversified steroidal saponin. However, the biological activities studies of these isolated steroidal saponins lag behind, especially in anti-inflammatory related activities.
Acknowledgements
This work was financially supported by the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences (No. P2015-KF07), Science and Technology Program of Guangzhou, China (No. 201607010147), and Guangdong Medical Science Foundation (No. A2015225).
Compliance with Ethical Standards
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Editorial Committee of the flora of the Chinese Aacdemy of Sciences, ‘Flora Reipublicae Popularis Sinicae’, Beijing House of Science Press (1978)
- 2.Chinese Pharmacopoeia Commission . Chinese Pharmacopoeia. Beijing: China Medical Science Press; 2015. [Google Scholar]
- 3.Compilation Group of Chinese Hebral Medicine . The Assembly of Chinese Herbal Medicine. Beijing: People’s Medical Publishing House; 1975. [Google Scholar]
- 4.Ma H, Wang W, Dong F. Zhongguo Yaofang. 2009;20:1191–1193. [Google Scholar]
- 5.Yi Y, Liu Y, Peng Y, Xie Y, Wang S. Zhongguo Yaofang. 2011;25:1430–1432. [Google Scholar]
- 6.Guo J, Qian F, Li J, Xu Q, Chen T. Clin. Chem. 2007;53:465–471. doi: 10.1373/clinchem.2006.077297. [DOI] [PubMed] [Google Scholar]
- 7.Bernardo RR, Pinto AV, Parente JP. Phytochemistry. 1996;43:465–469. doi: 10.1016/0031-9422(96)00274-9. [DOI] [PubMed] [Google Scholar]
- 8.Sashida Y, Kubo S, Mimaki Y, Nikaido T, Ohmoto T. Phytochemistry. 1992;31:2439–2443. doi: 10.1016/0031-9422(92)83634-B. [DOI] [PubMed] [Google Scholar]
- 9.Cho KY. Yakhak Hoechi. 1995;39:141–147. [Google Scholar]
- 10.Belhouchet Z, Sautour M, Miyamoto T, Lacaille-Dubois MA. Chem. Pharm. Bull. 2008;56:1324–1327. doi: 10.1248/cpb.56.1324. [DOI] [PubMed] [Google Scholar]
- 11.Challinor VL, Parsons PG, Chap S, White EF, Blanchfield JT, Lehmann RP, De Voss JJ. Steroids. 2012;77:504–511. doi: 10.1016/j.steroids.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Iskenderov GB, Mamedova MN, Musaev NI. Chem. Nat. Comp. 1975;11:820. doi: 10.1007/BF00568488. [DOI] [Google Scholar]
- 13.Ju Y, Jia ZJ. Phytochemistry. 1992;31:1349–1351. doi: 10.1016/0031-9422(92)80288-P. [DOI] [PubMed] [Google Scholar]
- 14.Ju Y, Jia ZJ. Phytochemistry. 1993;33:1193–1195. doi: 10.1016/0031-9422(93)85048-V. [DOI] [PubMed] [Google Scholar]
- 15.Ju Y, Peng HR, Jia ZJ, Sun XJ. J. Lanzhou Univ. (Nat. Sci.) 1994;30:64–67. [Google Scholar]
- 16.Sun XJ, Ju Y, Du M, Jia ZJ. Zhongcaoyao. 1995;26:395–396. [Google Scholar]
- 17.Ivanova A, Mikhova B, Klaiber I, Dinchev D, Kostova I. Nat. Prod. Res. 2009;23:916–924. doi: 10.1080/14786410802624827. [DOI] [PubMed] [Google Scholar]
- 18.Lin T, Huang HL, Liu RH, Shu JC, Ren G, Shao F, Liu LS. Magn. Reson. Chem. 2012;50:813–817. doi: 10.1002/mrc.3883. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Li X, Zhao CC, Wang Y. Nat. Prod. Res. 2008;22:884–889. doi: 10.1080/14786410701642557. [DOI] [PubMed] [Google Scholar]
- 20.Wu XH, Wang CZ, Wang SQ, Mi C, He Y, Zhang J, Zhang YW, Anderson S, Yuan CS. J. Ethnopharmacol. 2015;162:362–368. doi: 10.1016/j.jep.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C, Feng S, Zhang L, Ren Z. Nat. Prod. Res. 2013;27:1255–1260. doi: 10.1080/14786419.2012.725396. [DOI] [PubMed] [Google Scholar]
- 22.Khan I, Nisar M, Ebad F, Nadeem S, Saeed M, Khan H, Samiullah F, Khuda N Karim, Ahamd Z. J. Ethnopharmacol. 2009;121:175–177. doi: 10.1016/j.jep.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Kubo S, Mimaki Y, Sashida Y, Nikaido T, Ohmoto T. Phytochemistry. 1992;31:2445–2450. doi: 10.1016/0031-9422(92)80084-R. [DOI] [PubMed] [Google Scholar]
- 24.Hou PY, Mi C, He Y, Zhang J, Wang SQ, Yu F, Anderson S, Zhang YW, Wu XH. Fitoterapia. 2015;105:43–48. doi: 10.1016/j.fitote.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Sautour M, Miyamoto T, Lacaille-Dubois MA. J. Nat. Prod. 2005;68:1489–1493. doi: 10.1021/np058060b. [DOI] [PubMed] [Google Scholar]
- 26.Sautour M, Miyamoto T, Lacaille-Dubois MA. Planta Med. 2006;72:667–670. doi: 10.1055/s-2006-931582. [DOI] [PubMed] [Google Scholar]
- 27.Huang HL, Liu RH, Shao F. Magn. Reson. Chem. 2009;47:741–745. doi: 10.1002/mrc.2455. [DOI] [PubMed] [Google Scholar]
- 28.Xu J, Feng S, Wang Q, Cao Y, Sun M, Zhang C. Molecules. 2014;19:20975–20987. doi: 10.3390/molecules191220975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.da Silva BP, Parente JP. Z. Naturforsch. B. 2008;63:95–100. [Google Scholar]
- 30.Shao B, Guo H, Cui Y, Ye M, Han J, Guo D. Phytochemistry. 2007;68:623–630. doi: 10.1016/j.phytochem.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Ivanova A, Mikhova B, Batsalova T, Dzhambazov B, Kostova I. Fitoterapia. 2011;82:282–287. doi: 10.1016/j.fitote.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Liang F, He JW, Zhu GH, Liu RH, Shu JC, Shao F, Huang HL. Phytochem. Lett. 2016;16:294–298. doi: 10.1016/j.phytol.2016.05.011. [DOI] [Google Scholar]
- 33.Ju Y, Jia Z, Sun X. Phytochemistry. 1994;37:1433–1436. doi: 10.1016/S0031-9422(00)90427-8. [DOI] [PubMed] [Google Scholar]
- 34.Challinor VL, De Voss JJ. Nat. Prod. Rep. 2013;30:429–454. doi: 10.1039/c3np20105h. [DOI] [PubMed] [Google Scholar]
- 35.Yang CR, Zhang Y, Jacob MR, Khan SI, Zhang YJ, Li XC. Antimicrb. Agents. Chemother. 2006;50:1710–1714. doi: 10.1128/AAC.50.5.1710-1714.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shu XS, Gao ZH, Yang XL. J. Ethnopharmacol. 2006;103:327–332. doi: 10.1016/j.jep.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Laitonjam WS, Kongbrailatpam BD. Nat. Prod. Res. 2010;24:1168–1176. doi: 10.1080/14786410903040402. [DOI] [PubMed] [Google Scholar]
- 38.Tschesche R, Lüdke G, Wulff G. Tetrahedron Lett. 1967;8:2785–2790. doi: 10.1016/S0040-4039(00)90859-1. [DOI] [Google Scholar]
- 39.Ju Y, Jia ZJ, Peng HR, Sun XJ. Chin. Chem. Lett. 1993;4:137–138. [Google Scholar]
- 40.Ju Y, Jia ZJ. Chem. J. Chin. Univ. 1990;11:1386–1387. [Google Scholar]
- 41.Jia ZH, Ju Y, Du M. Chin. Chem. Lett. 1992;3:431–432. [Google Scholar]
- 42.Ju Y, Jia ZJ, Wu YN. Chin. Chem. Lett. 1991;2:853–854. [Google Scholar]
- 43.Zhang CL, Zhu W, Cheng YM, Li WC. Xibei Zhiwu Xuebao. 2003;23:1973–1976. [Google Scholar]
- 44.Zhang CL, Gao JM, Zhu W. Phytochem. Lett. 2012;5:49–52. doi: 10.1016/j.phytol.2011.09.005. [DOI] [Google Scholar]
- 45.Woo MH, Do JC, Son KH. J. Nat. Prod. 1992;55:1129–1135. doi: 10.1021/np50086a015. [DOI] [Google Scholar]
- 46.Zhang CL, Zhu W, Li XM, Su BF, Yan XY. Linye Kexue. 2006;42:69–73. [Google Scholar]
- 47.Jia ZH, Ju Y. Phytochemistry. 1992;31:3173–3175. doi: 10.1016/0031-9422(92)83469-F. [DOI] [PubMed] [Google Scholar]
- 48.Zhang CL, Li WC, Gao JM, Fu JX. J. Northwest Sci-Tech Univ. of Agric. For. (Nat. Sci.) 2003;31:163–166. [Google Scholar]
- 49.Ju Y, Jia ZJ. Chem. J. Chin. Univ. 1991;12:1488–1489. [Google Scholar]
- 50.Lavaud C. Planta Med. Phytother. 1993;26:64–73. [Google Scholar]
- 51.Liu JY, Fu JX, Gao JM, Qiu MH. J. Northwest Sci-Tech Univ. of Agric. For. (Nat. Sci.) 2002;30:222–224. [Google Scholar]
- 52.Chen W, Shou XA, Chen Y, Qin N, Qiao W, Tang SA, Duan HQ. Chem. Nat. Comp. 2014;50:989–993. doi: 10.1007/s10600-014-1143-1. [DOI] [Google Scholar]
- 53.Li J, Bi X, Zheng G, Hitoshi Y, Ikeda T, Nohara T. Chem. Pharm. Bull. 2006;54:1451–1454. doi: 10.1248/cpb.54.1451. [DOI] [PubMed] [Google Scholar]
- 54.Wu XH, Wang CZ, Zhang J, Wang SQ, Han L, Zhang YW, Yuan CS. Phytother. Res. 2014;28:1822–1828. doi: 10.1002/ptr.5207. [DOI] [PubMed] [Google Scholar]
- 55.Wu XH, Zhang J, Wang SQ, Yang VC, Anderson S, Zhang YW. Phytomedicine. 2014;21:1196–1201. doi: 10.1016/j.phymed.2014.03.009. [DOI] [PubMed] [Google Scholar]


