Abstract
The development of efficient transfection protocols for livestock cells is crucial for implementation of cell-based transgenic methods to produce genetically modified animals. We synthetized fully deacylated linear 22, 87 and 217 kDa polyethylenimine (PEI) nanoparticles and compared their transfection efficiency and cytotoxicity to commercial branched 25 kDa PEI and linear 58 kDa poly(allylamine) hydrochloride. We studied the effect of PEI size and presence of serum on transfection efficiency on primary cultures of bovine fetal fibroblasts and established cells lines (HEK 293 and Hep G2). We found that transfection efficiency was affected mainly by polymer/pDNA ratio and DNA concentration and in less extent by PEI MW. In bovine fibroblast, preincubation of PEI nanoparticles with fetal bovine serum (FBS) greatly increased percentage of cells expressing the transgene (up to 82%) while significantly decreased the polymer cytotoxic effect. 87 and 217 kDa PEI rendered the highest transfection rates in HEK 293 and Hep G2 cell lines (>50% transfected cells) with minimal cell toxicity. In conclusion, our results indicate that fully deacylated PEI of 87 and 217 kDa are useful DNA vehicles for non-viral transfection of primary cultures of bovine fetal fibroblast and HEK 293 and Hep G2 cell lines.
Keywords: Transgenesis, Cattle, Fibroblasts, Polyethylenimine, Cell transfection
Introduction
A broad range of potential applications of transgenic farm animals have been proposed, including disease modeling, recombinant protein production and improvement of productivity traits (Niemann et al. 2009). Somatic cell nuclear transfer (SCNT) has become a major tool for producing large transgenic farm animals. For SCNT, in vitro cultured cells are genetically manipulated and used as nuclear donor to generate transgenic offspring. Although somatic cells are not normally considered a limiting resource for SCNT, identification of cheap, efficient and low toxicity protocols for DNA delivery into cells would positively impact SCNT-based transgenic methodologies, particularly when working with hard-to-transfect or low prolificacy cell lines.
Polycations have been extensively used as non-viral alternative vectors for delivery of nucleic acids into cells for many decades (Boussif et al. 1995). One of the most studied polycations is PEI, which can be manufactured to achieve different sizes (kDa) and structures (linear or branched) depending on the particular synthesis process (Lázaro-Martínez et al. 2015; Thomas et al. 2005b). The high positive charge density of PEI at neutral pH could explain its capacity to condense plasmid DNA (pDNA) into polyplexes. These PEI/pDNA complexes are endocytosed by cells and transported through the cytoplasm to the nucleus, while preventing pDNA from enzymatic degradation during cellular trafficking and digestion by nucleases (Lee et al. 2010). The ability of PEI to form complexes with nucleic acids and carry them into the cell nucleus has been extensively documented under in vivo and in vitro conditions (cells in culture). However, a major drawback associated with the use of PEI as transfection reagent is the cytotoxic effect on exposed cells and tissues. A commonly reported finding is impaired cell viability after in vitro transfections with the polymer (Fan et al. 2012; Moghimi et al. 2005; Yang et al. 2008) and severe systemic side effects and inflammation in exposed animals (Chollet et al. 2002; Kawakami et al. 2006).
Several studies have connected transfection efficiency and cytotoxicity of PEI preparations to physicochemical properties, namely molecular weight and branching ratio of the polymer (Godbey et al. 1999; Mintzer and Simanek 2009; Neu et al. 2005; Ogris et al. 1998). Consequently, different strategies have been devised (i.e., PEI size, branch density, deacylation, and conjugation with functional molecules, among others) in order to reduce cell damage after PEI transfections. However, most of these techniques are labor intensive and/or require time consuming purification steps (Jiang and Salem 2012; Kichler 2004; Sawant et al. 2012; Swami et al. 2007; Yang et al. 2008).
The aim of this work was to study the effect of polymer size and polymer/pDNA ratio on bovine fetal fibroblast (BFF), HEK 293 and Hep G2 transfection efficiency and cytotoxicity. We synthetized linear 22, 87 and 217 kDa polymers (22K, 87K and 217K) and compared their transfection efficiency and cytotoxicity with a commercial branched 25 kDa PEI (25K) and a linear 58 kDa poly(allylamine) hydrochloride (58K PAH) in primary cultures of BFF and established cell lines.
Materials and methods
Polymer synthesis
The linear poly(ethyleneimine hydrochloride) (PEI) polymers with molecular weights of 22, 87 and 217 kDa were synthesized from the corresponding 50, 200 and 500 kDa poly(2-ethyl-2-oxazoline) (Sigma-Aldrich, St. Louis, MO, USA) respectively. Different molecular weight PEIs were synthetized as described by Lazaro Martinez et al. (2015) with minor modifications: 1 g of poly(2-ethyl-2-oxazoline) was added to 120 mL of a 24% HCl solution and refluxed for 96 h. The poly(2-ethyl-2-oxazoline) dissolved approximately in 1 h, and 2 h later a white precipitate (PEI) appeared in the flask and persisted throughout the rest of the reaction. Then, the reaction mixture was filtered and the white solid was washed with 5 mL of deionized water. Then, the solid was air-dried for 48 h, dissolved in 40 mL of deionized water and lyophilized overnight.
The linear poly(allylamine) hydrochloride (PAH) with an average molecular weight of 58 kDa and the branched 25 kDa PEI were purchased from Sigma-Aldrich. JetPRIME®, transfection reagent was purchased from Polyplus-transfection SA (Illkirch, France).
Physicochemical characterization of polymers
Identification and quality of the synthesized products were determined by 1H and 13C-NMR spectroscopy. 1H-NMR (D2O, 300 MHz) δ (pm): 3.58 (s, 2H) and 13C-NMR (D2O, 75 MHz) δ (pm): 42.7. A comprehensive physicochemical characterization of synthesized polymers can be found in a previous report (Lázaro-Martínez et al. 2015).
Agarose gel retardation assay
pDNA condensation with polymers was assessed by an electrophoretic mobility assay. For polymer-pDNA polyplex formation, a fixed amount of pDNA (3 μg) was mixed with increasing amounts of different polymers and incubated 10 min for complexation at room temperature in a humidity chamber. Samples were loaded on a 0.8% agarose gel containing a green fluorescent DNA dye (1:10,000; GelGreen, Biotium, Hayward, CA, USA), and run at 12 V/cm in TAE buffer for 40 min. Gels were observed in a blue light transilluminator and photographed. Then agarose gels were stained with Coomassie Brilliant Blue to reveal polymer nanoparticles.
Isolation of BFF and culture of BFF, HEK 293 and Hep G2 cells
Bovine fetal fibroblasts were obtained from slaughterhouse fetuses of 90–150 days of gestation. Fetuses were transported to the laboratory where they were processed in a laminar-flow cabinet. A piece of subdermal tissue (about 1 cm2) was removed from the flank of the fetus and sectioned with a scalpel blade into smaller pieces. Explants were placed in cell culture plates (3–4 explants per 100 mm plate) in 6 mL of cell culture medium (DMEM, 1 × antibiotic/antimycotic, Gibco, Carlsbad, CA, USA) supplemented with 10% FBS (Natocor, Cordoba, Argentina) and cultured for approximately 10 days at 38.5 °C in an atmosphere of 5% CO2 in air and high humidity. When the cells reached 70–80% confluence, they were trypsinized (trypsin 0.5%, Sigma-Aldrich Co.) and passaged to a T-75 culture flask for cell propagation. Fibroblasts were frozen in DMEM containing 20% FBS and 10% DMSO (Sigma-Aldrich Co.) and kept in liquid nitrogen until use.
Human embryonic kidney cells 293 (HEK 293, American Type Culture Collection, Manassas, VA, USA) and Hep G2 (human liver cancer cell line, American Type Culture Collection) were cultured in cell culture medium (DMEM, 1× antibiotic/antimycotic, Gibco, CA, USA) at 37 °C in an atmosphere of 5% CO2 in air and high humidity. When the cells reached 70–80% confluence, they were trypsinized (trypsin 0.5%, Sigma-Aldrich Co.) and passaged to a T-75 culture flask for cell propagation. Both cell types were frozen in DMEM containing 20% FBS and 10% DMSO (Sigma-Aldrich Co.) and kept in liquid nitrogen until use.
Transient in vitro gene transfection
Twenty-four hours prior to the gene transfections, BFF, HEK 293 and Hep G2 cells were plated in a 24-well plate at an initial density of 30,000 cells/well. pZsGreen1-N1 mammalian expression plasmid (Clontech Laboratories Inc., Mountain View, CA USA) was purified from E. coli DH5-α strain with ZymoPURE™ maxi prep kit (Zymo Research, Irvine, CA, USA) and used as reporter construct. This plasmid encodes a human codon-optimized variant of the wild-type Zoanthus sp. green fluorescent protein, ZsGreen1 under the control of the constitutive promoter human cytomegalovirus (CMV) immediate early promoter.
For transfection assays, each polymer solution was adjusted to 1 μg/μL, pH 7 and passed through a 0.22 µm pore size filter and stored in sterile 1.5 mL tubes. Polymer samples were subjected to three freeze–thaw cycles (−80 to 37 °C) before used in transfection experiments.
Polymer/pDNA polyplexes for cell transfection were prepared at different ratios (w/w) by adding an appropriate amount of PEI to 0.5, 1 or 2 μg of pZsGreen1-N1 in 300 μL of DMEM plus antibiotics with or without 10% fetal bovine serum (FBS), depending on particular experimental design. These polymer ratios were chosen based on our previous work, in which we reported optimal conditions for transfection of BFF with commercial PEI 25K (Forcato et al. 2012). The polymer/pDNA mixtures were incubated for 15 min at room temperature before use to allow for complexation.
After 3 h of incubation of cells with polyplexes, 700 µL of DMEM + FBS medium were added to each well. Reporter gene expression was determined 48 h after transfection in a Becton–Dickinson FACScan flow cytometer (Becton–Dickinson, Franklin Lakes, NJ, USA).
The JetPRIME/pDNA complexes were prepared according to the manufacturer´s recommendations.
Stable transgene integration
In order to establish if PEI nanoparticles could mediate stable transgene integration into cell´s genome, BFF, HEK 293 and Hep G2 cell cultures were transfected with the components of the Sleeping Beauty transposon system as previously described for bovine cells (Alessio et al. 2016). Briefly, the three cell types were cotransfected with a helper plasmid (pCMV-SB100X) which carries an expression cassette for the SB transposase, and two donor vectors (pT2/Venus/RMCE which harbors an expression cassette for Venus fluorescent protein, and pT2/SV40-Neo carrying a neo resistance cassette; cassettes in both plasmids are flanked by the SB inverted terminal repeats). All plasmids were purified from E. coli DH5-α strain with ZymoPURE™ maxi prep kit (Zymo Research). After 21-days antibiotic selection, monoclonal colonies were individually recovered from 100 mm plates using cloning rings. Monoclonal colonies were trypsinized and transferred to 100 mm plates to obtain monoclonal transgenic cell lines. Genomic DNA was isolated from transgenic cell lines using Quick-gDNA™ MiniPrep (Zymo Research) following the manufacturer’s protocol. Transgene integration was assessed by PCR amplification of a 280-pb fragment from the Venus gene using the following primers: Fw: TAGCCCAGGGTGGTCACCAG; Rev: TGTGACCGGCGGCTCTAGAG.
MTT assay
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) reduction assay was used to study the effect of polymers on cell viability. BFF, HEK 293 or Hep G2 cells were seeded in a 96-well plate at an initial density of 5000 cells/well in 100 μL of the DMEM +10% SFB. After 24 h, the cells were exposed to increasing concentration of polymers and incubated in a humidified environment with 5% CO2 at 38.5 or 37 °C depending on each cell type requirements. Then, MTT reagent (5 mg/mL) was added to each well 24 h after polymers addition and incubated for 3 h. The medium in each well was removed and replaced by 100 μL of DMSO. The absorbance at 540 nm was recorded by a microplate reader (Bio-Rad, Hercules, CA, USA). Each treatment was run in octuplicates. The cell viability is expressed as percentage of change over the control (considered 100%) and it was calculated with the following formula: Cell viability (%) = (OD of polymer-treated sample/OD of untreated sample) × 100. The data are shown as the mean value ± SEM.
Results
Polymer/pDNA complexation analysis
Polymer/pDNA complexation is known to be prerequisite for cell internalization and successful transfection (Tian et al. 2007). Formation of polymer/pDNA complexes was studied by agarose gel electrophoresis. Complexation was inferred from the retardation of pDNA mobility in the gel.
As it can be seen in Fig. 1, PEI 22K, 87K, and 217K and the commercial PEI 25K efficiently complexed pDNA at almost the same PEI/pDNA ratios (no free pDNA mobility was observed at these ratios). Critical PEI/pDNA ratio for complete retardation was similar for all PEIs under study, since for both PEI 22K and 87K it was about 1.7 (Fig. 1a, c, respectively) and for PEI 25K and 217K was about 2.0 (Fig. 1b, d). Complete pDNA retardation with PAH was not observed at the PAH/pDNA ratios studied, what may explain the poor transfection efficiency of PAH observed in our study (see below).
Fig. 1.
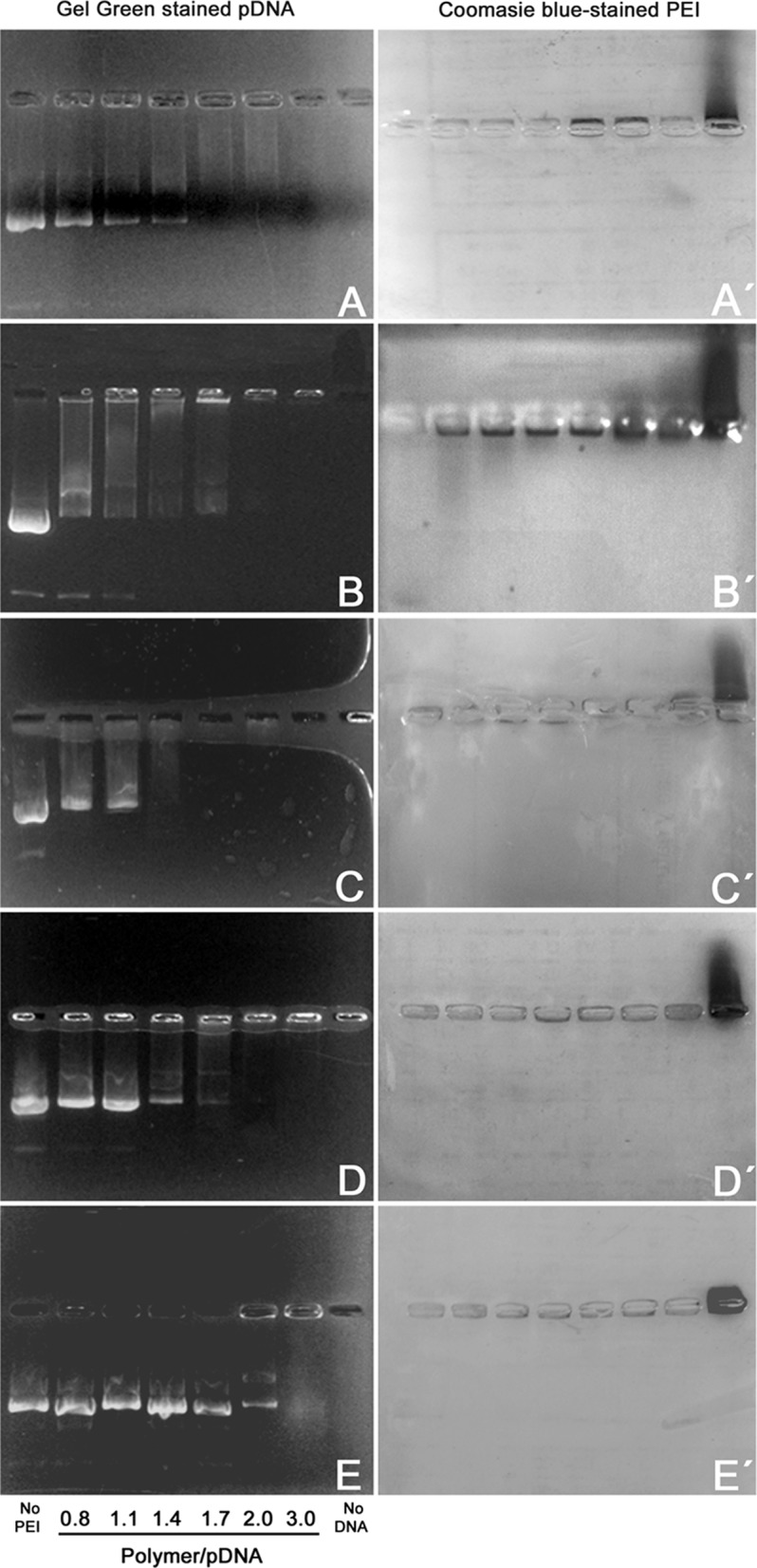
Agarose gel electrophoresis of polymer particles mixed at varying ratios with circular pDNA: 0.8, 1.1, 1.4, 1.7, 2.0 or 3.0. pDNA (3 μg) and polymer alone (5 μg) controls were also included. a PEI 22K. b PEI 25K. c PEI 87K. d PEI 217K. e PAH 58K. Gel photographs on the left show Gel Red stained DNA migration observed under blue LED light. Pictures on the right (a′ through e′) correspond to the same agarose gels stained with Coomassie Brilliant Blue to reveal polymer electrophoretic migration. Reverse migration of polymers alone can be observed on the rightmost lane in each gel
Polymer cytotoxic effect on BFF
It has long been known that cationic polymer MW affects both transfection efficiency and cytotoxicity (Fischer et al. 1999). Low MW PEIs offer poor transfection efficiency and are nontoxic or slightly toxic to exposed cells both in vitro and in vivo (Lungwitz et al. 2005; Neu et al. 2005). Conversely, both acylated and deacylated high MW PEIs are efficient for delivering DNA into cells but cause significant cytotoxicity, mainly at high concentrations. High concentration of polymeric cations in the extracellular medium has been reported to be harmful to different cells lines (Thomas et al. 2005a, b). Here we determined cytotoxicity of homemade fully deacylated PEIs (22, 87, and 217 kDa), 25 kDa commercial PEI and PAH on BFF, HEK 293 and Hep G2 cells.
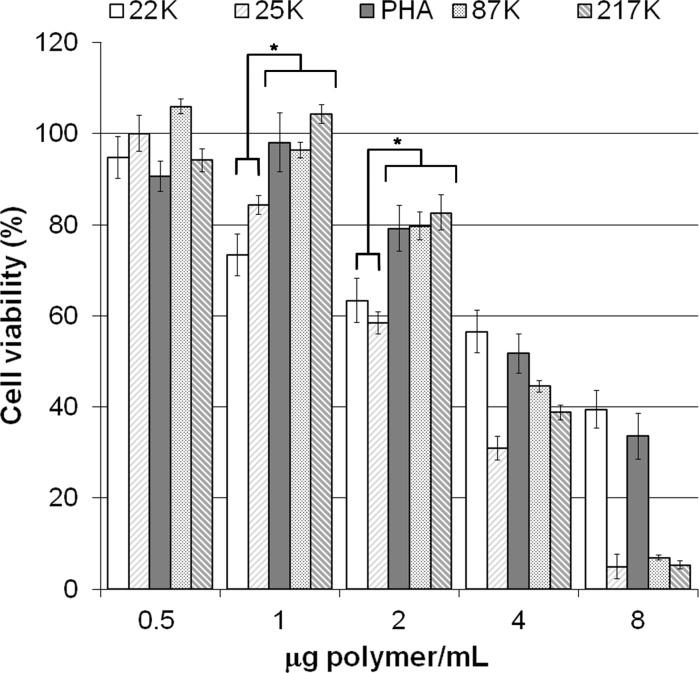
For all studied polymers, BFF viability decreased as the polymer concentration increased (Fig. 2). No cytotoxic effect was detected when cells were exposed to 0.5 μg/ml of PEIs or PAH. Cell viability of cells incubated with 1 μg/mL of PAH, PEI 87K or 217K was not affected by the treatment, but the same concentration of PEI 22K and 25K significantly impaired BFF viability (Fig. 2; p < 0.05). Similarly, at 2 μg/ml cell viability was compromised in cultures treated with PEI 22K and 25K (Fig. 2). PEI 22K and PAH were the less cytotoxic polymers, although their transfection efficiency was poor (Table 1). At 4 μg/mL, all polymers were highly cytotoxic since cell viability dropped below 60%.
Fig. 2.
Dose-dependence effect of cationic polymers in DMEM without FBS or pDNA on BFF viability. Cell viability was measured by MTT metabolism. Results are expressed as a percentage of the absorbance of untreated cells and presented as the mean of three independent replicates ±SEM. Viability of cells treated with PEI 22K and 25K at 1 and 2 µg of polymer per ml was significantly lower compared to the other polymers studied (asterisk denotes differences at p < 0.05)
Table 1.
Transfection efficiency (percentage of fluorescent cells) obtained with different polymer/pDNA ratios (1:1 and 2:1) in DMEM without FBS
| Polymer/pDNA ratio (w/w) | 1:1 | 2:1 | |||
|---|---|---|---|---|---|
| pDNA (μg) | 2 | 1 | 0.50 | 1 | 0.50 |
| PEI 22K | 2.5 | 0.7 | 0.8 | 21.2 | 2.6 |
| PEI 25K | 32.5 | 13.5 | 1.2 | 38.4 | 12.2 |
| PEI 87K | 1.1 | 0.5 | 0.6 | 24.0 | 2.7 |
| PEI 217K | 13.0 | 7.3 | 0.6 | 22.1 | 15.1 |
| PAH | 16.2 | 2.5 | 2.3 | 7.9 | 2.3 |
Effect of serum on BFF transfection efficiency
We hypothesized that presence of serum during cell transfection reduces polymer cytotoxicity and enhances transfection efficiency. Therefore, BFF cultures were transfected with different polymers in absence or presence of 10% FBS and the efficiency of gene delivery determined by flow cytometry. Two different polymer to pDNA ratios were used, i.e., 1:1 and 2:1. Transfection efficiency ranged from 0.5 to 38.4% GFP positive cells, with the best efficiencies obtained with 2 μg of polymer complexed with 1 µg of pDNA (Table 1).
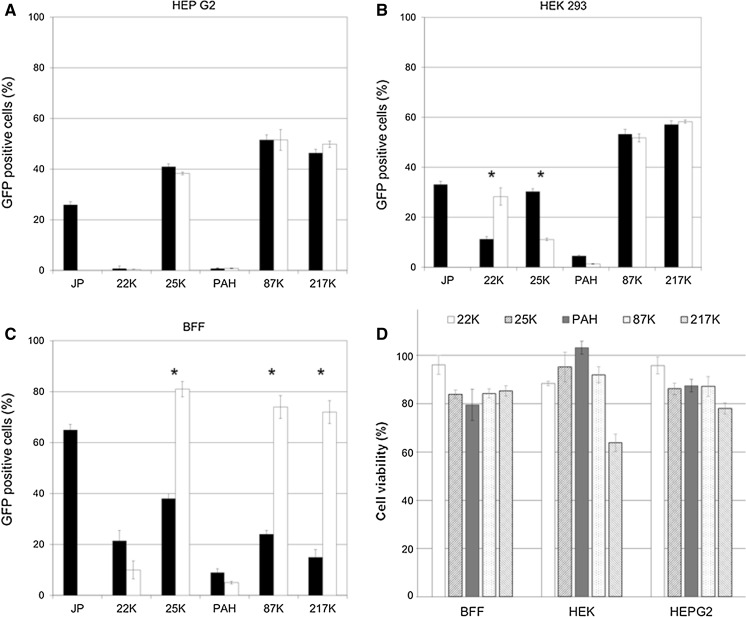
Carrabino et al. (2005) reported that complexation of PEI with pDNA in presence of human serum albumin increased transfection efficiency and markedly decreased polymer toxicity. Since human albumin is difficult to purify and therefore expensive, we decided to use fetal bovine serum (FBS) as a homologous protein source. Polymer/pDNA ratio of 2:1 using 1 μg pDNA, which assures a balance of considerable transfection efficiency and acceptable toxicity (cell survival >60% for all polymers studied), was chosen to further investigate the effects of FBS on cell toxicity and transfection. Presence of FBS during the complexation step significantly increased cell transfection efficiency for fully deacylated PEI 87K and PEI 217K and for branched 25K, while it failed to increase efficiency of gene delivery for 22K and PAH (Fig. 3).
Fig. 3.
a–c Effect of preincubation of different sized polyplexes (polymer/pDNA at ratio 2:1) in DMEM alone or supplemented with 10% FBS (DMEM + FBS) on transfection efficiency (DMEM, black bars; DMEM + FBS, white bars) in Hep G2 and HEK 293 cell lines and BFF. Asterisk denote significant differences (p< 0.05) between DMEM versus DMEM + SBF within each PEI treatment. d Effect of different sized polymers without pDNA at 2 µg polymer/ml in DMEM + FBS on cell viability measured by MTT assay. The data are presented as the mean ± SEM from three independent replicates. Results are expressed as a percentage of the absorbance of untreated cells and presented as the mean of three independent replicates ±SEM
Interestingly, the commercial branched PEI 25K, which is known to possess a higher charge density than the fully deacylated linear PEIs (Jeong et al. 2001), showed a higher cytotoxic effect compared to 87K and 217K when incubated without FBS, supporting the idea that a higher charge density contributes to the toxic effect due to stronger electrostatic interactions with anionic macromolecules from the cell surface as some authors have suggested (Di Gioia and Conese 2009; Hunter 2006).
However, PEI 22K, which possesses the lower charge density per molecule, caused as much reduction on cell viability as non deacylated 25K, suggesting that particle size could be also related to polymer cytotoxicity. The addition of FBS to small MW PEIs (deacylated or not) could increase significantly polyplexes size, and therefore reduce cell toxic effect of small-PEIs. This effect is clearly observed in Fig. 3d, where PEI 22K and 25K polyplexes assembled in DMEM + FBS improved cell viability to values above 80%.
Transfection and cytotoxicity studies in HEK 293 and Hep G2 cell lines
In order to extend the study to other cell lines, we replicated the same experiments performed with BFF, in HEK 293 and Hep G2, two established human-derived cell lines commonly used as experimental model. PEI 87K and 217K resulted in higher transfection efficiency than commercial agent and PEI 25K. Addition of FBS did not enhance transfection rates in HEK 293 and Hep G2 cell lines (Fig. 3a, b), as it did for BFF. Presence of FBS did not increase cell toxicity in these cell lines, showing cell viability values around or over 80% in all cases except when HEK 293 was treated with PEI 217K, where cell viability dropped to 63.8 ± 3.6% (Fig. 3d), a value comparable to BFF viability treated with PEI 22K and 25K without FBS (Fig. 2).
Stable transgene integration
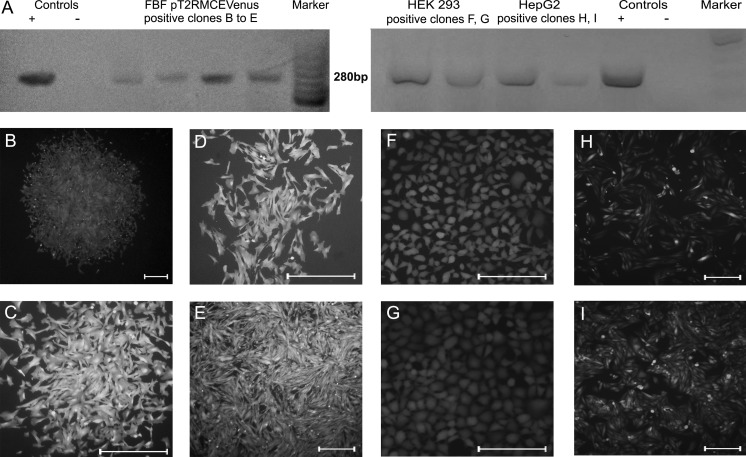
To assess stable transgene integration using PEI 25K as pDNA vehicle, BFF, HEK 293 and Hep G2 were cotransfected with the SB plasmids (donors and helper) and selected with G418 during 21 days. Genomic DNA from resistant colonies (Fig. 4b–i) was isolated for PCR genotyping. Fluorescent colonies from BFF (Fig. 4a–d), HEK 293 (Fig. 4f, g) and Hep G2 (Fig. 4h, i) were positive for Venus sequence in their genomes (Fig. 4a) demonstrating that PEI-delivered pDNA is released from endosomes and can be integrated into the cell genome.
Fig. 4.
a PCR analysis of genomic DNA from SB-transgenic monoclonal cell lines. Venus amplicon (280 pb product) originated from genomic DNA from four BFF (left panel), two HEK 293 and two Hep G2 (right panel) randomly selected clonal lines. C+ = positive controls. C− = negative controls. MW markers: GeneRuler 50 bp DNA Ladder (Thermo Scientific; left panel) and 100 bp DNA Ladder (Invitrogen™, right panel). Fluorescence microscopy images of four monoclonal BFF colonies (b–e) transfected with the Sleeping Beauty system and selected during 21 days with G418. Fluorescence microscopy images of two HEK 293 (f, g) and two Hep G2 monoclonal colonies (h, i) transfected with the Sleeping Beauty system and selected during 21 days with G418. Bar = 100 μm
Discussion
In this work we performed the optimization of BFF transfection conditions and a comparative analysis of the transfection efficiency and cytotoxicity of homemade, fully deacylated linear PEIs (22K, 87K and 217K) with commercially available 25K branched PEI and PAH 58K, a fully deacylated polymer chemically related to PEI in BFF primary cultures and in two frequently used cell lines, HEK 293 and Hep G2. The results show that as DNA concentration increases the transfection efficiency until it reaches a plateau and that gene delivery efficiency of different sized PEI was dependent on PEI/pDNA ratio. PEI/pDNA ratio of 2:1 led to the highest transfection efficiency for all PEIs included in this study.
Interestingly, 87K and 217K polyplexes exhibited similar high transfection efficiency as PEI/pDNA 25K in BFF transfected with FBS-supplemented medium. Transfection of HEK 293 and Hep G2 with PEI 87K and 217K was associated with high transgene incorporation rates regardless of presence FBS, transfection rates that were significantly larger compared with those obtained with the commercial agent and 25K PEI.
We also included in the study PAH, which is a polymer chemically related to PEI but by far less studied as gene delivery agent (Zhao et al. 2012). We found that PAH showed lower cytotoxicity than PEIs, however its transfection efficiency was poor. PAH lower toxicity allowed us to test a 2:1 ratio using 4 μg of PAH and 2 μg of pDNA), which resulted in a fair transfection efficiency of 34.5 ± 2.7%. Despite the comparatively low transfection efficiency of PAH, its low toxicity allows the use of higher amounts of pDNA per transfection assay, which can be useful in cotransfection experiments where two or more plasmids must be delivered into cells.
To maximize the transfection efficiency, PEI/pDNA ratio is known to play a crucial role by influencing the size and the surface charge of the PEI/pDNA polyplexes, since small cationic particles mediate binding to anionic cell surface proteoglycans triggering cell endocytosis (Huh et al. 2007; Rhaese et al. 2003). The commercial linear PEI 25K was less toxic than its fully deacylated variant, suggesting that a higher charge density contributes to the toxic effect, presumably due to stronger electrostatic interactions with cellular anionic macromolecules.
An optimal polymer/pDNA ratio that warrant that no pDNA nor polymer remains free, increases the efficacy of DNA complexation making polyplexes more prone to cellular internalization via endocytosis (Kichler 2004). Excess polymer should be avoided since uncomplexed free fraction may impair cytoplasmic membrane function (Fischer et al. 1999). Furthermore, the presence of PEI in the cell nucleus might interfere with transcriptional processes (Godbey et al. 2001) eventually leading to the cell death. Our results suggest that lower cytotoxicity observed in FBS preincubated polyplexes could be related to adsorption of free PEI to serum proteins.
On the other hand, high cell PEI/pDNA uptake is desirable for 2 reasons: as we show in this work, more plasmid implies a higher transfection rate (both transient and stable) and a higher amount of PEI inside the endosome may be crucial for modification and permeabilization of endosomal membrane for efficient delivery of pDNA into the cytoplasm (Liang and Lam 2012; Moghimi et al. 2005). Since increasing PEI MW implies more water adsorption, a higher MW PEI should be more desirable in order to maximize these effects.
We found considerable cytotoxicity in increasing high MW PEI concentrations in absence of FBS. On the contrary, exposure of cells to polycations in presence of FBS reduced cytotoxicity in the cell types studied, supporting the idea that FBS preincubation of polyplexes provides a new way to modify PEI, enhancing its gene transfection efficiency without compromising cell viability.
All these findings highlight the importance of an exact PEI/pDNA ratio plus a putative protective effect of FBS, presumably due its albumin content, although other serum proteins and lipoproteins could be implicated in this phenomenon. The exact mechanism by which FBS exerts its cell protective effect remains to be elucidated, but possible explanations are (1) adsorption of harmful free PEI by serum proteins or (2) quenching of positive surface charge of polyplexes that are therefore less harmful to cellular membranes (Tros de Ilarduya et al. 2010). It is important to note that the MTT assay is not indicative of apoptosis as this may occur at later stages depending on cell type. Regarding BFF cells, whose performance in PEI transfection is reported here for the first time, cytometric analysis provide no evidence of apoptosis after 24 h of incubation with PEI (data not shown).
In conclusion, here we demonstrated the effectivity of diverse MW PEIs on transfection of fetal fibroblast primary cultures and on two established routinely used cell lines. We show that high MW deacylated PEIs (87K and 217K) can lead to high-frequency transient transfection in BFF as in other broadly used cell lines like HEK 293 and Hep G2, and are able to generate stable transfected lines under selective pressure when polyplexes are formed in presence of FBS.
The addition of FBS to the PEI/pDNA mixture make it possible to achieve cost-effective transfection of BFF, establishing a foundation to utilize transfected fibroblasts in gene expression studies as well as to generate transgenic cattle through SCNT.
Acknowledgements
This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET) and Universidad Nacional de Río Cuarto (UNRC), República Argentina.
Funding
Funding was provided by CONICET (PIP 2012-2014 (114 201101 00278)), MinCyT (PICT-2012-0514).
Compliance with ethical standards
Conflict of interest
None of the authors have any conflict of interest to declare.
Footnotes
D. O. Forcato and A. E. Fili have contributed equally to this work.
References
- Alessio AP, Fili AE, Garrels W, Forcato DO, Olmos Nicotra MF, Liaudat AC, Bevacqua RJ, Savy V, Hiriart MI, Talluri TR, Owens JB, Ivics Z, Salamone DF, Moisyadi S, Kues WA, Bosch P. Establishment of cell-based transposon-mediated transgenesis in cattle. Theriogenology. 2016;85:e1292. doi: 10.1016/j.theriogenology.2015.12.016. [DOI] [PubMed] [Google Scholar]
- Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrabino S, Di Gioia S, Copreni E, Conese M. Serum albumin enhances polyethylenimine-mediated gene delivery to human respiratory epithelial cells. J Gene Med. 2005;7:1555–1564. doi: 10.1002/jgm.799. [DOI] [PubMed] [Google Scholar]
- Chollet P, Favrot MC, Hurbin A, Coll JL. Side-effects of a systemic injection of linear polyethylenimine-DNA complexes. J Gene Med. 2002;4:84–91. doi: 10.1002/jgm.237. [DOI] [PubMed] [Google Scholar]
- Di Gioia S, Conese M. Polyethylenimine-mediated gene delivery to the lung and therapeutic applications. Drug Des Devel Ther. 2009;2:163–188. doi: 10.2147/dddt.s2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Wu X, Ding B, Gao J, Cai Z, Zhang W, Yin D, Wang X, Zhu Q, Liu J, Ding X, Gao S. Degradable gene delivery systems based on Pluronics-modified low-molecular-weight polyethylenimine: preparation, characterization, intracellular trafficking, and cellular distribution. Int J Nanomed. 2012;7:1127–1138. doi: 10.2147/IJN.S27117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, Bieber T, Li Y, Elsässer HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res. 1999;16:1273–1279. doi: 10.1023/A:1014861900478. [DOI] [PubMed] [Google Scholar]
- Forcato DO, Olmos Nicotra MF, Ortega NM, Alessio AP, Fili AE, Rodríguez N, Bosch P. Optimization of branched 25 kDa polyethylenimine for efficient gene delivery in bovine fetal fibroblasts. Reprod Fertil Dev. 2012;25:313. doi: 10.1071/RDv25n1Ab331. [DOI] [Google Scholar]
- Godbey WT, Wu KK, Mikos AG. Size matters: molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J Biomed Mater Res. 1999;45:268–275. doi: 10.1002/(SICI)1097-4636(19990605)45:3<268::AID-JBM15>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Godbey WT, Wu KK, Mikos AG. Poly(ethylenimine)-mediated gene delivery affects endothelial cell function and viability. Biomaterials. 2001;22:471–480. doi: 10.1016/S0142-9612(00)00203-9. [DOI] [PubMed] [Google Scholar]
- Huh SH, Do HJ, Lim HY, Kim DK, Choi SJ, Song H, Kim NH, Park JK, Chang WK, Chung HM, Kim JH. Optimization of 25 kDa linear polyethylenimine for efficient gene delivery. Biologicals. 2007;35:165–171. doi: 10.1016/j.biologicals.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Hunter AC. Molecular hurdles in polyfectin design and mechanistic background to polycation induced cytotoxicity. Adv Drug Deliv Rev. 2006;58:1523–1531. doi: 10.1016/j.addr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Jeong JH, Song SH, Lim DW, Lee H, Park TG. DNA transfection using linear poly(ethylenimine) prepared by controlled acid hydrolysis of poly(2-ethyl-2-oxazoline) J Control Release. 2001;73:391–399. doi: 10.1016/S0168-3659(01)00310-8. [DOI] [PubMed] [Google Scholar]
- Jiang D, Salem AK. Optimized dextran-polyethylenimine conjugates are efficient non-viral vectors with reduced cytotoxicity when used in serum containing environments. Int J Pharm. 2012;427:71–79. doi: 10.1016/j.ijpharm.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami S, Ito Y, Charoensit P, Yamashita F, Hashida M. Evaluation of proinflammatory cytokine production induced by linear and branched polyethylenimine/plasmid DNA complexes in mice. J Pharmacol Exp Ther. 2006;317:1382–1390. doi: 10.1124/jpet.105.100669. [DOI] [PubMed] [Google Scholar]
- Kichler A. Gene transfer with modified polyethylenimines. J Gene Med. 2004;6:S3–S10. doi: 10.1002/jgm.507. [DOI] [PubMed] [Google Scholar]
- Lázaro-Martínez JM, Rodríguez-Castellón E, Vega D, Monti GA, Chattah AK. Solid-state Studies of the crystalline/amorphous character in linear poly(ethylenimine hydrochloride) (PEI·HCl) polymers and their copper complexes. Macromolecules. 2015;48:1115–1125. doi: 10.1021/ma5023082. [DOI] [Google Scholar]
- Lee K, Bae KH, Lee Y, Lee SH, Ahn CH, Park TG. Pluronic/polyethylenimine shell crosslinked nanocapsules with embedded magnetite nanocrystals for magnetically triggered delivery of siRNA. Macromol Biosci. 2010;10:239–245. doi: 10.1002/mabi.200900291. [DOI] [PubMed] [Google Scholar]
- Liang W, Lam JKW. Endosomal escape pathways for non-viral nucleic acid delivery systems. In: Ceresa B, editor. Molecular regulation of endocytosis. Croatia: InTech; 2012. pp. 421–467. [Google Scholar]
- Lungwitz U, Breunig M, Blunk T, Göpferich A. Polyethylenimine-based non-viral gene delivery systems. Eur J Pharm Biopharm. 2005;60:247–266. doi: 10.1016/j.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev. 2009;109:259–302. doi: 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- Moghimi SM, Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A. A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol Ther. 2005;11:990–995. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Neu M, Fischer D, Kissel T. Recent advances in rational gene transfer vector design based on poly(ethylene imine) and its derivatives. J Gene Med. 2005;7:992–1009. doi: 10.1002/jgm.773. [DOI] [PubMed] [Google Scholar]
- Niemann H, Kues W, Carnwath JW. Transgenic farm animals: current status and perspectives for agriculture and biomedicine. In: Engelhard M, Hagen K, Boysen M, editors. Genetic engineering in livestock. Berlin: Springer; 2009. pp. 1–30. [Google Scholar]
- Ogris M, Steinlein P, Kursa M, Mechtler K, Kircheis R, Wagner E. The size of DNA/transferrin-PEI complexes is an important factor for gene expression in cultured cells. Gene Ther. 1998;5:1425–1433. doi: 10.1038/sj.gt.3300745. [DOI] [PubMed] [Google Scholar]
- Rhaese S, von Briesen H, Rübsamen-Waigmann H, Kreuter J, Langer K. Human serum albumin-polyethylenimine nanoparticles for gene delivery. J Control Release. 2003;92:199–208. doi: 10.1016/S0168-3659(03)00302-X. [DOI] [PubMed] [Google Scholar]
- Sawant RR, Sriraman SK, Navarro G, Biswas S, Dalvi RA, Torchilin VP. Polyethyleneimine-lipid conjugate-based pH-sensitive micellar carrier for gene delivery. Biomaterials. 2012;33:3942–3951. doi: 10.1016/j.biomaterials.2011.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swami A, Aggarwal A, Pathak A, Patnaik S, Kumar P, Singh Y, Gupta KC. Imidazolyl-PEI modified nanoparticles for enhanced gene delivery. Int J Pharm. 2007;335:180–192. doi: 10.1016/j.ijpharm.2006.11.033. [DOI] [PubMed] [Google Scholar]
- Thomas M, Ge Q, Lu JJ, Chen J, Klibanov AM. Cross-linked small polyethylenimines: while still nontoxic, deliver DNA efficiently to mammalian cells in vitro and in vivo. Pharm Res. 2005;22:373–380. doi: 10.1007/s11095-004-1874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Lu JJ, Ge Q, Zhang C, Chen J, Klibanov AM. Full deacylation of polyethylenimine dramatically boosts its gene delivery efficiency and specificity to mouse lung. Proc Natl Acad Sci USA. 2005;102:5679–5684. doi: 10.1073/pnas.0502067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Xiong W, Wei J, Wang Y, Chen X, Jing X, Zhu Q. Gene transfection of hyperbranched PEI grafted by hydrophobic amino acid segment PBLG. Biomaterials. 2007;28:2899–2907. doi: 10.1016/j.biomaterials.2007.02.027. [DOI] [PubMed] [Google Scholar]
- Tros de Ilarduya C, Sun Y, Düzgüneş N. Gene delivery by lipoplexes and polyplexes. Eur J Pharm Sci. 2010;40:159–170. doi: 10.1016/j.ejps.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Yang Z, Sahay G, Sriadibhatla S, Kabanov AV. Amphiphilic block copolymers enhance cellular uptake and nuclear entry of polyplex-delivered DNA. Bioconjug Chem. 2008;19:1987–1994. doi: 10.1021/bc800144a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao E, Zhao Z, Wang J, Yang C, Chen C, Gao L, Feng Q, Hou W, Gao M, Zhang Q. Surface engineering of gold nanoparticles for in vitro siRNA delivery. Nanoscale. 2012;4:5102–5109. doi: 10.1039/c2nr31290e. [DOI] [PubMed] [Google Scholar]