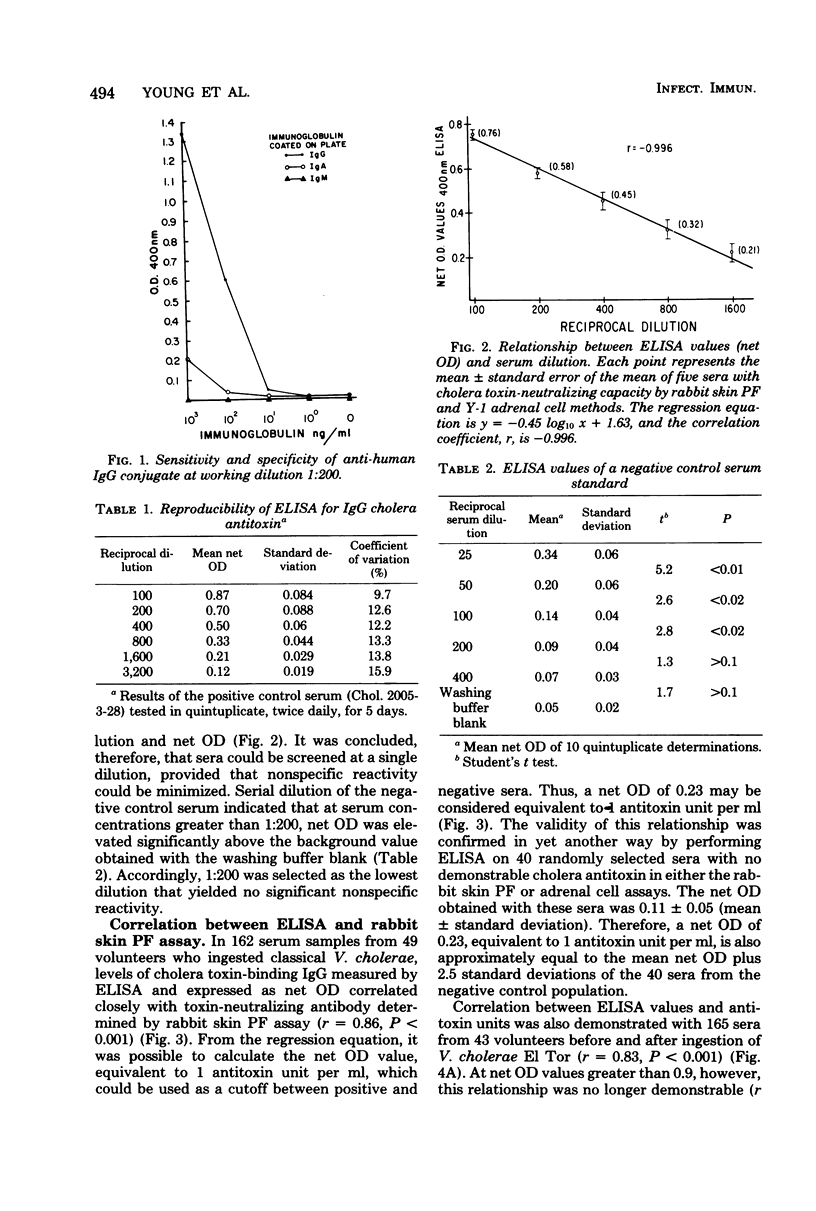
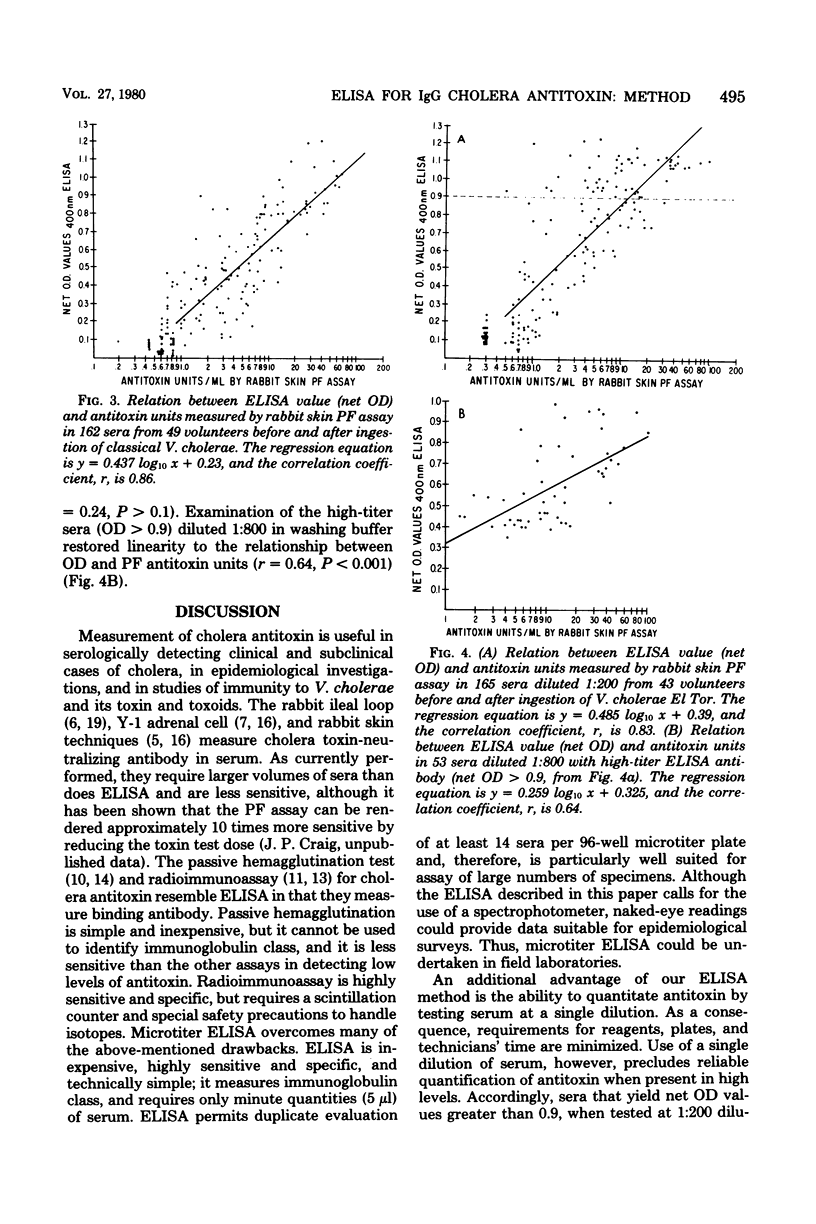
Abstract
A microtiter enzyme-linked immunosorbent assay (ELISA) to measure immunoglobulin G cholera antitoxin in human serum has been developed. The ELISA employs commercially available reagents, including cholera enterotoxin and goat anti-human immunoglobulin G. It is specific, sensitive, and reproducible and requires as little as 5 microliter of serum. ELISA, moreover, permits quantitative determination of cholera antitoxin at a single serum dilution of 1:200. A total of 162 pre- and post-challenge sera from 49 volunteers who ingested Vibrio cholerae classical biotype, and 165 sera from 43 volunteers who ingested V. cholerae El Tor biotype, were tested for cholera antitoxin by ELISA and by the rabbit skin vascular permeability factor assay. The correlation between the two assays was statistically significant (P less than 0.001). ELISA for immunoglobulin G cholera antitoxin thus provides a valuable in vitro correlate of in vivo toxin-neutralizing capacity. Microtiter ELISA permits duplicate evaluation of at least 14 sera per 96-well plate including blanks and controls, is readily adapted to use in field studies, and therefore is particularly well suited to seroepidemiological surveys.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benenson A. S., Saad A., Mosley W. H., Ahmed A. Serological studies in cholera. 3. Serum toxin neutralization--rise in titre in response to infection with Vibrio cholerae, and the level in the "normal" population of East Pakistan. Bull World Health Organ. 1968;38(2):287–295. [PMC free article] [PubMed] [Google Scholar]
- Cash R. A., Music S. I., Libonati J. P., Snyder M. J., Wenzel R. P., Hornick R. B. Response of man to infection with Vibrio cholerae. I. Clinical, serologic, and bacteriologic responses to a known inoculum. J Infect Dis. 1974 Jan;129(1):45–52. doi: 10.1093/infdis/129.1.45. [DOI] [PubMed] [Google Scholar]
- Clem T. R., Yolken R. H. Practical colorimeter for direct measurement of microplates in enzyme immunoassay systems. J Clin Microbiol. 1978 Jan;7(1):55–58. doi: 10.1128/jcm.7.1.55-58.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. P., Eichner E. R., Hornick R. B. Cutaneous responses to cholera skin toxin in man. I. Responses in unimmunized American males. J Infect Dis. 1972 Mar;125(3):203–215. doi: 10.1093/infdis/125.3.203. [DOI] [PubMed] [Google Scholar]
- Curlin G. T., Craig J. P., Subong A., Carpenter C. C. Antitoxic immunity in experimental canine cholera. J Infect Dis. 1970 May;121(5):463–470. doi: 10.1093/infdis/121.5.463. [DOI] [PubMed] [Google Scholar]
- Donta S. T. Neutralization of cholera enterotoxin-induced steroidogenesis by specific antibody. J Infect Dis. 1974 Mar;129(3):284–288. doi: 10.1093/infdis/129.3.284. [DOI] [PubMed] [Google Scholar]
- Donta S. T., Sack D. A., Wallace R. B., Dupont H. L., Sack R. B. Tissue-culture assay of antibodies to heat-liable Escherichia coli enterotoxins. N Engl J Med. 1974 Jul 18;291(3):117–121. doi: 10.1056/NEJM197407182910302. [DOI] [PubMed] [Google Scholar]
- Engvall E. Quantitative enzyme immunoassay (ELISA) in microbiology. Med Biol. 1977 Aug;55(4):193–200. [PubMed] [Google Scholar]
- Finkelstein R. A., Peterson J. W. In vitro detection of antibody to cholera enterotoxin in cholera patients and laboratory animals. Infect Immun. 1970 Jan;1(1):21–29. doi: 10.1128/iai.1.1.21-29.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Levine M. M., Merson M. H., Sack R. B., Sack D. A., Valdesuso J. R., Nalin D., Hoover D., Chanock R. M., Kapikian A. Z. Solid-phase microtiter radioimmunoassay blocking test for detection of antibodies to Escherichia coli heat-labile enterotoxin. J Clin Microbiol. 1979 Jan;9(1):60–64. doi: 10.1128/jcm.9.1.60-64.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyles C. L. Immunological study of the heat-labile enterotoxins of Escherichia coli and Vibrio cholerae. Infect Immun. 1974 Mar;9(3):564–570. doi: 10.1128/iai.9.3.564-570.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejtmancik K. E., Peterson J. W., Markel D. E., Kurosky A. Radioimmunoassay for the antigenic determinants of cholera toxin and its components. Infect Immun. 1977 Sep;17(3):621–628. doi: 10.1128/iai.17.3.621-628.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein H. D., Feeley J. C., DeWitt W. E. Titration of cholera antitoxin in human sera by microhemagglutination with formalinized erythrocytes. Appl Microbiol. 1970 May;19(5):742–745. doi: 10.1128/am.19.5.742-745.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M. Enzyme-linked immunosorbent assays for cholera serology. Infect Immun. 1973 May;7(5):759–763. doi: 10.1128/iai.7.5.759-763.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Hughes T. P., Young C. R., O'Donnell S., Craig J. P., Holley H. P., Bergquist E. J. Antigenicity of purified glutaraldehyde-treated cholera toxoid administered orally. Infect Immun. 1978 Jul;21(1):158–162. doi: 10.1128/iai.21.1.158-162.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Nalin D. R., Craig J. P., Hoover D., Bergquist E. J., Waterman D., Holley H. P., Hornick R. B., Pierce N. P., Libonati J. P. Immunity of cholera in man: relative role of antibacterial versus antitoxic immunity. Trans R Soc Trop Med Hyg. 1979;73(1):3–9. doi: 10.1016/0035-9203(79)90119-6. [DOI] [PubMed] [Google Scholar]
- Nalin D. R., al-Mahmud A., Curlin G., Ahmed A., Peterson J. Cholera toxoid boosts serum Escherichia coli antitoxin in humans. Infect Immun. 1974 Oct;10(4):747–749. doi: 10.1128/iai.10.4.747-749.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


