Abstract
BACKGROUND
The purpose of this multicenter pilot study was to: (1) determine the frequency of regional cerebral oxygen saturation (rSco2) desaturations during cardiac surgery involving cardiopulmonary bypass (CPB); (2) evaluate the accuracy of clinician-identified rSco2 desaturations compared with those recorded continuously during surgery by the near-infrared spectroscopy (NIRS) monitor; and (3) assess the effectiveness of an intervention algorithm for reversing rSco2 desaturations.
METHODS
Two hundred thirty-five patients undergoing coronary artery bypass graft and/or valvular surgery were enrolled at 8 US centers in this prospective observational study. NIRS (Invos™ 5100C; Covidien) was used to monitor rSco2 during surgery. The frequency and magnitude of rSco2 decrements >20% from preanesthesia baseline were documented, and the efficacy of a standard treatment algorithm for correcting rSco2 was determined. The data from the NIRS monitor were downloaded at the conclusion of surgery and sent to the coordinating center where the number of clinician-identified rSco2 desaturation events was compared with the number detected by the NIRS monitor.
RESULTS
The average rSco2 obtained at baseline (mean ± SD, 61% ± 11%; 99% confidence interval, 57%–65%) and during CPB (62% ± 14%; 57%–67%) was not different. However, rSco2 after separation from CPB (56% ± 11%; 53%–60%) was lower than measurements at baseline and during CPB (P < 0.001). During CPB, rSco2 desaturations occurred in 61% (99% confidence interval, 50%–75%) of patients. The area under the curve for product of magnitude and duration of the rSco2 was (mean ± SD, 145.2; 384.8% × min). Clinicians identified all patients with an rSco2 desaturation but identified only 340 (89.5%) of the 380 total desaturation events. Of the 340 clinician-identified rSco2 desaturation events, 115 resolved with usual clinical care before implementation of the treatment algorithm. For the remaining 225 events, the treatment algorithm resulted in resolution of the rSco2 desaturation in all but 18 patients.
CONCLUSIONS
This multicenter pilot study found that 50% to 75% of patients undergoing cardiac surgery experience one or more rSco2 desaturations during CPB. Nearly 10% of desaturation events were not identified by clinicians, suggesting that appropriate alarming systems should be adopted to alert clinicians of such events. The intervention algorithm was effective in reversing clinically identified rSco2 desaturations in the majority of events.
Near-infrared spectroscopy (NIRS) is used increasingly during cardiac surgery to monitor regional cerebral oxygen saturation (rSco2) as an indicator of cerebral perfusion adequacy, particularly during cardio-pulmonary bypass (CPB).1,2 Unlike pulse oximetry, NIRS monitoring does not distinguish arterial from venous blood. Because approximately 70% to 75% of cerebral blood volume is venous blood, rSco2 provides an indicator of O2 supply versus-demand balance.3 Case reports and observational studies have linked episodes of rSco2 desaturation during coronary artery bypass graft (CABG) surgery with risk for stroke and postoperative cognitive dysfunction.1,2 These studies, although, have limitations that confound the ability to interpret whether decrements in rSco2 during surgery are clearly linked with adverse neurologic outcomes.1,2 Low rSco2 may merely serve as an epiphenomenon identifying patients with cerebral vascular disease or limited cardio-pulmonary reserve who are at high risk of complications.4 Importantly, limited data are available on the effectiveness of interventions aimed at correcting rSco2 desaturations and whether such interventions lead to improved patient neurologic outcomes.5–10
Many clinicians, administrators, and health care payers may desire a higher level of evidence than what is currently available on the benefits of NIRS monitoring before it is adopted for routine use in patients undergoing cardiac surgery. Such evidence would ideally come from multicenter prospective studies that compare the effect of interventions to correct rSco2 desaturations with the effect of current standard of care on the frequency of neurologic complications. As a first step to achieve this goal, in this pilot study, we assembled a group of investigators at 8 US institutions to: (1) determine the frequency of rSco2 desaturations during cardiac surgery with CPB; (2) compare the accuracy of clinician-identified rSco2 desaturations with that of continuous NIRS monitoring during surgery; and (3) assess the effectiveness of an intervention algorithm for reversing rSco2 desaturations. We hypothesized that rSco2 desaturations are frequent during CPB and that a multicenter network of investigators can successfully follow an intervention algorithm and accurately report their data to a central data-coordinating center. As a proof of concept construct, the intent of the study was not to link desaturations or their correction to specific outcomes. However, these data may be of value to clinicians for establishing reference ranges of rSco2 and for evaluating potential interventions for treating rSco2 desaturations.
METHODS
The procedures of this study were approved by The Johns Hopkins Medical Institutions Human Research Review Board. The procedures further met with the approval of the IRBs of 7 other enrolling sites: Beth Israel Deaconess Hospital, Harvard Medical School, Boston, MA; Brigham and Women’s Hospital/Cape Cod Hospital, Harvard Medical School, Hyannis, MA; Duke University School of Medicine, Durham, NC; Emory University School of Medicine, Atlanta, GA; Mayo University School of Medicine, Rochester, MN; University of Virginia School of Medicine, Charlottesville, VA; and University of Maryland School of Medicine, Baltimore, MD. All study procedures were performed only after receiving individual written informed patient consent.
Data Collection and Patient Monitoring
From November 8, 2013, to March 4, 2015, 239 patients were enrolled at the 8 study sites; this analysis focuses on 235 patients with nonmissing demographic and medical history data. Male and female patients were eligible for inclusion if they were >50 years old and undergoing primary or reoperative CABG and/or cardiac valve surgery. Enrolled patients at each research site were assigned a sequential 3-digit study number and a 4-character alpha code. Study data were collected on a paper case report form provided by the coordinating center (The Johns Hopkins University School of Medicine). The collected data included preoperative demographics and medical history. Medical conditions such as hypertension, prior stroke or transient ischemic attack, congestive heart failure, and other diseases were based on diagnosis by the patient’s primary medical team.
The patients received institutionally standard anesthesia and surgery care, including direct arterial blood pressure monitoring. Before the induction of general anesthesia, the patients were connected to an NIRS monitor (Invos™ 5100C; Covidien, Boulder, CO). The forehead was cleansed with an alcohol swab, and 2 self-adhesive probes were attached according to manufacturer’s guidelines. Baseline rSco2 readings were obtained, while the patients were breathing room air. Nonpulsatile CPB flow was maintained between 2.0 and 2.4 L/min/m2. The patients were managed with α-stat pH management. There was no standardized algorithm for blood transfusion.
rSco2 Data Collection and Intervention Algorithm
An episode of rSco2 desaturation was defined as a >20% decline from baseline detected from either the right or the left sensor. During surgery, the time of any rSco2 desaturation was recorded on the study data sheet. Management of these rSco2 desaturations followed an algorithm as proposed by Murkin et al.11 and Denault et al.12 (Fig. 1). Clinicians and perfusionists were instructed on the study procedures and the treatment algorithm for rSco2 desaturation. They then recorded each sequential intervention and the time when the rSco2 desaturation was corrected. Subsequent rSco2 desaturations were similarly treated and recorded when they occurred. At the conclusion of the surgery, rSco2 data files from the NIRS monitor were downloaded to a USB driver.
Figure 1.
Treatment algorithm for regional cerebral oxygen saturation (rSco2) desaturations (>20% decline from baseline) during cardiopulmonary bypass. Adopted from Denault et al.12 CPB = cardiopulmonary bypass; MAP = mean arterial pressure.
Study demographic and other data were recorded onto the case report forms, which was then transferred to an Excel spreadsheet (Microsoft, Inc., Redmond, WA) format. Once completed, the electronic case report form was uploaded onto an Internet, study-specific, encrypted Web site supported by MedDium, Inc. (Bethesda, MD, https://www.meddium.com/). The rSco2 data from the NIRS monitor were also uploaded to the same Web site. Only deidentified data for a patient assigned a study number and alpha code were uploaded. The study data could be reviewed only by authorized personnel at the coordinating center.
Sample Size Calculation and Data Analysis
The primary outcome of this pilot study was the combined frequency of rSco2 desaturations from all enrolling centers. The frequency of this event is not known and was the aim of this study. We sought to enroll 28 patients at each site or a total of 225 patients. We added an additional 14 patients to this sample size as a buffer in case any protocol was incomplete. This sample size was chosen to be similar to that reported by Murkin et al.11 who observed rSco2 desaturation in 56% of 200 patients. In that study, the interventions to correct the rSco2 desaturations were effective in 80.4% of episodes. Thus, we expected 126 episodes of rSco2 desaturation during surgery in this pilot study.
Demographic, prior medical, and preoperative data are presented as mean ± SD or median (range) for continuous variables and frequencies for categorical variables and compared using 1-way analysis of variance, Kruskal-Wallis, or χ2 tests across the study sites. Pairwise comparisons after significant overall test were performed considering multiple comparisons using the Tukey method. The overall proportion of desaturation events was estimated for the entire sample and for each site. Ninety-nine percent confidence intervals (CI) are reported for all estimates; they were calculated using generalized linear models with site-specific robust variance estimates to account for potential nonindependence of observations within sites. rSco2 at baseline, the start of CPB, and the end of CPB was summarized and compared using generalized linear model with time (start and end of CPB versus baseline) as the primary independent variable. The model was estimated using generalized estimating equations with exchangeable working correlation structure to account for within-patient correlation of rSco2 levels over time. We evaluated the accuracy of clinician-identified rSco2 desaturations by comparing the number and the frequency of investigator-reported rSco2 saturations to the number recorded continuously by the NIRS monitor during surgery. The severity of rSco2 desaturations was determined as the area under the curve of magnitude and duration of rSco2 desaturations. The latter was determined off-line at the coordinating center from the uploaded rSco2 data. The number and specific type of each sequential intervention for rSco2 desaturation were determined. Compliance with the intervention algorithm for rSco2 desaturation was defined as a clinician’s successful correction of each rSco2 desaturation episode identified by retrospective review of the patient’s NIRS monitor data file.
RESULTS
Data were successfully collected from 235 patients. The medical and operative characteristics of the enrolled patients are shown in Table 1 and the Supplemental Table 1 (Supplemental Digital Content, http://links.lww.com/AA/B408). There were differences among the enrolling sites in some characteristics, including patient age, race, history of congestive heart failure, type of surgical procedure, and duration of CPB and aortic cross-clamping.
Table 1.
Patients’ Medical History and Operative Data
| Characteristic | n = 235 | P value between sites |
|---|---|---|
| Agea (y) | 68.3 ± 9.1 | 0.036 |
| Male/femaleb | 161 (68.5)/74 (31.5) | 0.117 |
| Heighta (cm) | 171.5 ± 9.6 | 0.792 |
| Weighta (kg) | 85.6 ± 20 | 0.043 |
| Raceb | <0.001 | |
| Caucasian | 204 (86.8) | |
| African American | 25 (10.6) | |
| Asian | 3 (1.3) | |
| Prior strokeb | 17 (7.2) | 0.160 |
| Prior transient ischemic attackb | 11 (4.7) | 0.154 |
| Hypertensionb | 180 (76.6) | 0.800 |
| Diabetesb | 72 (30.6) | 0.921 |
| Insulinb | 25 (10.6) | 0.922 |
| Chronic obstructive pulmonary diseaseb | 31 (13.2) | 0.806 |
| Congestive heart failureb | 63 (26.8) | <0.001 |
| Tobacco smokingb | 0.414 | |
| Current | 20 (8.5) | |
| Former | 108 (46.0) | |
| Atrial fibrillationb | 49 (20.9) | 0.213 |
| Prior myocardial infarctionb | 57 (24.3) | 0.249 |
| Medicationb | ||
| Aspirin | 181 (77.0) | 0.319 |
| Clopidogrel | 30 (12.8) | 0.108 |
| Prasugrel | 1 (0.4) | |
| Ticagrelor | 3 (1.3) | |
| Coumadin | 19 (8.1) | 0.862 |
| β-adrenergic receptor blockers | 171 (72.8) | 0.081 |
| Angiotensin-converting enzyme inhibitor | 78 (33.2) | 0.524 |
| Surgical procedureb | ||
| CABG surgery | 129 (54.9) | 0.003 |
| 1-vessel | 24 (10.2) | 0.038 |
| 2-vessel | 27 (11.5) | |
| 3-vessel | 49 (20.9) | |
| 4-vessel | 27 (11.5) | |
| 5-vessel | 2 (0.9) | |
| Aortic valve replacement | 85 (36.2) | 0.777 |
| Mitral valve replacement/repair | 61 (26.0) | 0.008 |
| Tricuspid valve replacement/repair | 13 (5.5) | 0.020 |
| Prior cardiac surgery | 24 (10.2) | 0.006 |
| Duration of CPB (min)c | 102.0 (31.0–329) | 0.002 |
| Duration of aortic cross-clamping (min)c | 74.0 (0–242) | 0.110 |
| Patients at each enrolling siteb | ||
| Beth Israel Deaconess Hospital | 28 (11.9) | |
| Cape Cod Hospital | 28 (11.9) | |
| Duke University Hospital | 28 (11.9) | |
| Emory University Hospital | 28 (11.9) | |
| Johns Hopkins Hospital | 37 (15.7) | |
| Mayo Clinic | 28 (11.9) | |
| University of Maryland Hospital | 30 (12.9) | |
| University of Virginia | 28 (11.9) | |
CABG = coronary artery bypass graft; CPB = cardiopulmonary bypass.
Mean ± SD.
Number (%).
Median (range).
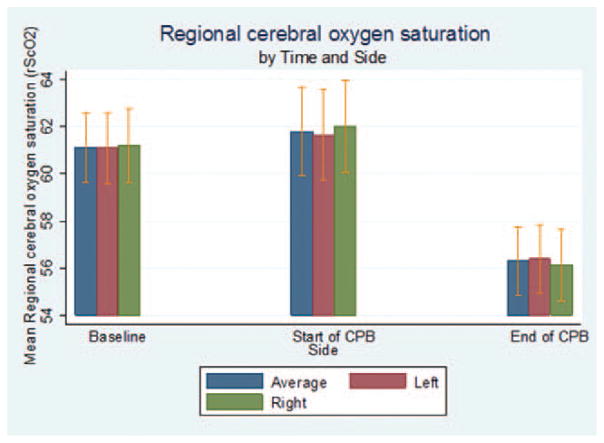
Baseline rSco2 and results obtained during and after separation from CPB are shown in Figure 2. Average baseline rSco2 for the left, right, and the average of the left and right frontal lobes, respectively, were (mean ± SD; 99% CI) 61% ± 11% (57%–65%), 61% ± 12% (58%–65%), and 61% ± 11% (57%–67%). During CPB, the average rSco2 measurements were 62% ± 14% (57%–66%), 62% ± 15% (57%–67%), and 62% ± 14% (57%–67%) for the left, right, and the average of the left and right sides, respectively. After CPB, these results were 56% ± 11% (53%–60%), 56% ± 12% (52%–60%), and 56% ± 11% (53%–60%). The average rSco2 during CPB was no different than baseline (P = 0.338), but rSco2 after separation from CPB was lower than that at baseline and that recorded during CPB after adjustment for site (P < 0.001). rSco2 values differed among enrolling sites. The average rSco2 was lower at Duke University Hospital than that at the Mayo Clinic before (P < 0.001), during (P = 0.001), and after CPB (P = 0.004). The average rSco2 also was lower at Duke University Hospital than that at The Johns Hopkins Hospital before CPB (P = 0.001). Thirty-one (15%; 99% CI, 5%–41%) patients had baseline left-sided rSco2 <50%, and 34 (15%; 99% CI, 6%–37%) had baseline right-sided rSco2 <50%, while breathing room air.
Figure 2.
Regional cerebral oxygen saturation (rSco2) at baseline, the start of cardiopulmonary bypass (CPB), and the end of CPB. The data (mean ± SD) were combined from all enrolling sites.
The frequency of rSco2 desaturations during CPB for all patients, as well as that observed at each enrolling site, is shown in Table 2. One or more rSco2 desaturations occurred in 61% (99% CI, 50%–75%) of patients during CPB. A total of 380 desaturation events were detected from data downloaded from the NIRS monitor at the conclusion of surgery. The frequency of desaturation varied among enrolling sites (P = 0.023). After adjusting for multiple comparisons, we identified a difference in the frequency of rSco2 desaturation between the Mayo Clinic and Duke (P = 0.004), Emory University and the Mayo Clinic (P = 0.027), and the Mayo Clinic and the University of Virginia (P = 0.032). Clinicians detected all patients with at least 1 rSco2 desaturation during CPB, but they identified 340 (89.5%) of the 380 desaturation events.
Table 2.
The Number and Frequency of One or More rSco2 Desaturations (>20% Decline from Baseline) and the Area Under the Curve (AUC) for Cumulative Duration of Desaturations (% × min) Occurring During Cardiopulmonary Bypass
| Total rSco2desaturations | Unilateral desaturation | Bilateral desaturation | Left AUCa | Right AUCa | Left and right AUCa | |
|---|---|---|---|---|---|---|
| Total number of patients with at least one event | 138 (61%) | 193 | 187 | 140.7 (358.0) | 149.7 (462.9) | 145.2 (384.8) |
| Beth Israel Deaconess | 14 (50%) | 20 | 19 | 74.3 (164.6) | 30.8 (66.6) | 52.6 (97.7) |
| Cape Cod Hospital | 15 (54%) | 14 | 13 | 141.8 (321.9) | 138.2 (303.3) | 140.0 (308.4) |
| Duke University | 18 (64%) | 26 | 28 | 106.2 (288.2) | 90.0 (134.2) | 98.1 (193.4) |
| Emory University | 23 (82%) | 31 | 36 | 173.2 (281.8) | 137.1 (187.9) | 155.1 (202.1) |
| Johns Hopkins | 23 (79%) | 34 | 25 | 199.2 (313.5) | 239.4 (459.6) | 219.3 (328.3) |
| Mayo Clinic | 12 (43%) | 25 | 19 | 107.6 (174.6) | 71.1 (142.8) | 89.4 (156) |
| University of Maryland | 15 (54%) | 18 | 24 | 219.3 (754) | 235.4 (819.7) | 227.3 (785.3) |
| University of Virginia | 18 (64%) | 25 | 23 | 101.1 (218.8) | 250.0 (811.1) | 175.5 (510.1) |
Mean (SD).
rSco2 = Regional cerebral oxygen saturation
The effectiveness of each intervention for reversing rSco2 desaturation is shown in Table 3. There was spontaneous resolution of the desaturation in 115 events. For the remaining 225 events, treating hypotension was the most effective strategy for correcting rSco2 desaturation. Desaturations remained unresolved during CPB in 18 (5%; 99% CI, 3%–10%) of the 340 events. Of the patients with resolved desaturations, 1 intervention was needed in 70 events, 2 interventions in 57 events, 3 interventions in 28, and >3 interventions were needed in 65 events.
Table 3.
Efficacy of Interventions to Correct Decrements in Regional Cerebral Oxygen Saturation (rSco2) of >20% from Baseline for the 340 Clinician-Identified Events
| Intervention-corrected rSco2 desaturation | |
|---|---|
| Treat hypotension | 67 (29.8%) |
| Increase Fio2% | 35 (15.6%) |
| Normalize CPB flow | 32 (14.2%) |
| RBC transfusion | 31 (13.8%) |
| Decrease CPB “Sweep Speed” | 25 (11.1%) |
| Deepen anesthesia | 24 (10.7%) |
| Adjust CPB cannula | 18 (8.0%) |
| Reposition head to midline | 6 (2.7%) |
There was resolution of 115 desaturation events with usual clinical care and not the treatment algorithm. The data are listed as the number of desaturation of events that were not spontaneously corrected with percentage based on the event effectiveness versus all events that responded to any intervention (n = 225). In 18 (8%) patients, the rSco2 was unresolved despite interventions.
CPB = cardiopulmonary bypass; RBC = red blood cell.
DISCUSSION
In this study, we observed that the average rSco2 during CPB was no different from that at baseline. However, average rSco2 decreased after separation from CPB compared with values measured at baseline and during CPB. Desaturation in rSco2 occurred in 61% (99% CI, 50%–75%) of patients. NIRS monitoring recorded 380 desaturation events, of which clinicians identified 340 (89.5%). In all but 5% (99% CI, 3%–10%) of patients, there was either resolution of rSco2 desaturations with usual clinical care or they were corrected by the intervention algorithm.
Many clinicians have embraced rSco2 monitoring as a means for judging the adequacy of cerebral perfusion during CPB in an effort to reduce the risk for adverse neurologic events. Currently, only a low level of evidence supports the use of rSco2 monitoring for this purpose. In a previously published systematic review, our group identified 43 articles involving 6399 patients that evaluated the relationship between rSco2 monitoring and adverse neurologic outcomes after cardiac surgery.1 The identified citations included 13 case reports and 27 observational studies. These reports provided anecdotal evidence that rSco2 desaturations can be indicative of rare CPB cannula malposition, allowing for timely surgical adjustments. Although the data were not consistent, some observational studies linked rSco2 desaturations during CPB and postoperative cognitive dysfunction or stroke. Interpretation of these studies, however, is confounded by small sample sizes, the use of a limited psychometric testing battery in many of the studies, the lack of patient testing after hospital discharge, use of historical controls, the failure to risk adjust in the data analysis, and the use of monitors from multiple manufacturers.
In our systematic review, we identified 2 studies in which patients were prospectively randomized to an intervention to treat rSco2 desaturations during CPB.11,13 Poor adherence to the intervention protocol in the study by Slater et al.,13 however, limits interpretation of those results. Murkin et al.11 randomly assigned 200 patients undergoing CABG surgery to 2 groups. In one, patients with rSco2 desaturations >30% below baseline were treated with a stepped intervention algorithm. These patients were compared with a control group for whom rSco2 data were collected but not displayed to clinicians. In that study, the patients in the control group had longer rSco2 desaturations (P = 0.014), a higher frequency of a composite organ complication end point (death, ventilation >48 hours, stroke, myocardial infarction, return for re-exploration [P = 0.048], and longer hospitalization in the intensive care unit [P=0.029]) than did patients in the intervention arm. Episodes of rSco2 desaturation were observed in 56 of 100 patients in the intervention group, and the success rate of the rSco2 desaturation intervention was 80.4%. The study by Murkin et al. did not have adequate power to detect a difference in neurologic complications. Moreover, the sample size calculations assumed a complication rate in the control arm of 40%, whereas the observed rate was 30%.
A higher level of evidence than is currently available seems desirable to justify routine rSco2 monitoring for the approximately 600,000 patients who undergo CABG and/or valvular surgery annually in the United States.14 Its use may be justified given the devastating consequences of stroke and cognitive complications after cardiac surgery, including high costs of care for affected patients. For example, postoperative stroke alone adds an average of 15 days to the length of hospitalization after cardiac surgery, increases hospital costs by >$35,000, and increases the likelihood of discharge to an extended care facility after surgery.15–17 In this study, we demonstrated the frequency of rSco2 desaturations, the feasibility of multicenter collection of rSco2 monitoring data, and the application of an intervention algorithm for desaturations. These results would be useful in the design of a comparative effectiveness trial that evaluates the cost-benefit relationship of rSco2 monitoring in patients undergoing cardiac surgery.
There is no universal definition of rSco2 desaturation. Widely reported definitions are based largely on older data obtained from patients undergoing carotid endarterectomy surgery. In those studies, decrements in rSco2 between 5% and 20% from baseline or values <50% ipsilateral to carotid artery cross-clamping were associated with reductions in transcranial Doppler–measured cerebral blood flow velocity, electroencephalogram slowing, alterations in somatosensory-evoked potentials, or mental status changes when surgery was performed under regional anesthesia.1 In this study, we defined rSco2 desaturation as a >20% decline from baseline but did not use a threshold value such as decrements <50%. Many patients have baseline rSco2 <50% that may result from factors other than acute reduction in cerebral oxygenation, such as low cardiac output, pulmonary disease, cerebral vascular disease, and anemia. Indeed, in this study, baseline rSco2 <50% was observed on the left side in 13% of patients and on the right side in 14% of patients, before induction of anesthesia. We observed a baseline averaged rSco2 of 61% ± 11% (99% CI, 57%–65%). In a single-center study of 250 CABG surgery patients, the reported ranges of rSco2 before cardiac surgery varied between 47% and 83%.5 In a larger prospective study of 1178 patients, preoperative room air rSco2 ranged from 57% to 67% for survivors but 47% to 62% for nonsurvivors after CABG surgery.4 We did observe differences in the baseline rSco2 value among enrolling sites that might be explained by differences in patient age, race, or preoperative medical conditions among the centers. Differences in the frequency of desaturation and greater responsiveness to interventions in our study compared with those reported by Murkin et al.11 might be explained, in part, by the different definitions of desaturation (>20% from baseline versus >30% decrement).
In our study, clinicians were not blinded to rSco2 data. We observed that there was resolution of the clinician-identified rSco2 desaturation in 115 (34%) of 340 events with usual clinical care. We did not include a threshold duration in our definition of desaturation. Thus, many of these events with resolution before implementation of the treatment algorithm were likely of short duration. Clinicians might have further intervened with corrective maneuvers (e.g., increasing CPB flow after transient reduction) before institution of the algorithm. Furthermore, our intent was to simulate a usual clinical environment of NIRS monitoring to enhance the external validity of our findings. Thus, we did not specify a protocol for setting the alarms on the NIRS monitor. Regardless, the treatment of hypotension was the most effective intervention at reversing rSco2 desaturations. In the randomized study by Murkin et al., raising blood pressure was effective in 62% of cases, and increasing CPB flow was the second most effective intervention.18 We did not record which medications were used to raise blood pressure. Drugs with vasoconstrictive properties potentially could influence rSco2 readings indirectly as a result of their effects on the scalp circulation. This potential effect is related to the imprecise subtraction algorithms used by NIRS monitors, whereby light absorption from superficial tissue is subtracted from absorption in deeper tissue to yield frontal lobe rSco2.1,19,20 Hypothetically, vasoconstriction of scalp blood vessels could reduce rSco2 readings regardless of any benefit of higher blood pressure on cerebral blood flow. However, in this investigation, we observed correction of rSco2 desaturation with raised blood pressure. Regardless of these theoretical concerns, investigations during carotid endarterectomy surgery found that changes in rSco2 were 87.5% sensitive and 100% specific for identifying changes in transcranial Doppler blood flow velocity during selective clamping of the internal carotid artery. At the same time, rSco2 monitoring had 0% sensitivity and specificity for identifying laser Doppler blood flow velocity of the scalp during selective clamping of the external carotid artery.21
We observed that 57% (i.e., 193 of 340) of clinician-detected rSco2 desaturations were unilateral. This observation may explain why 3% of desaturation events responded to a repositioning of the head to the midline. Presumably, head position away from the midline might impede cerebral venous return and reduce cerebral perfusion pressure. Malposition of CPB venous or arterial cannulae might also explain some cases of unilateral rSco2 desaturation. Repositioning of CPB cannulae was effective in 8% of cases. The most effective interventions for rSco2 desaturation collectively were those aimed at improving cerebral oxygen delivery included increasing the FIo2% of gas delivery, avoiding the cerebral vasoconstrictive effects of hypocapnia by decreasing gas flow to the membrane oxygenator (i.e., decreasing “sweep speed”), ensuring appropriate CPB flow, and increasing oxygen-carrying capacity with red blood cell transfusion. These efforts were effective in 55% of desaturation events that were not spontaneously corrected with usual clinical care.
In this study, we did not relate rSco2 desaturations or their treatment to patient outcomes. The small sample size and limited funding available did not permit our team to collect such outcomes. Regardless, this study was meant as a pilot to establish feasibility for performing such a study. Finally, our definition of desaturation did not include the duration that rSco2 was >20% below baseline. Although short and long desaturations were considered equally, we did quantify severity by calculating area under the curve of events.
In conclusion, in this multicenter pilot study, we found that between 50% and 75% of patients undergoing cardiac surgery experience one or more rSco2 desaturations during CPB. Nearly 10% of desaturation events were not identified by clinicians, suggesting that appropriate alarming systems should be adopted to alert clinicians of such events. Acute rSco2 desaturation events either spontaneously corrected with usual clinical care or in response to the intervention algorithm in all but 18 (5%) of 340 clinician-identified events.
Acknowledgments
Funding: Supported, in part, by an unrestricted grant from Covidien, Inc. (Medtronic/Covidien, Boulder, CO), by individual institutional funds, and by National Institutes of Health (NIH) grant R01 94600 to Dr. Hogue. We would like to acknowledge support for the statistical analysis from the National Center for Research Resources and the National Center for Advancing Translational Sciences (NCATS) of the NIH through grant number 1UL-1TR001079.
Footnotes
Conflict of Interest: See Disclosures at the end of the article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.anesthesia-analgesia.org).
Reprints will not be available from the authors.
DISCLOSURES
Name: Balachundhar Subramanian, MD.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Conflicts: Balachundhar Subramanian reported no conflicts of interest.
Attestation: Balachundhar Subramanian has seen the original study data, reviewed the analysis of the data, and is the author responsible for archiving the study files.
Name: Charles Nyman, BS.
Contribution: This author helped conduct the study, analyze the data, and write the manuscript.
Conflicts: Charles Nyman reported no conflicts of interest. Attestation: Charles Nyman has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Name: Maria Fritock, MD.
Contribution: This author helped conduct the study, analyze the data, and write the manuscript.
Conflicts: Maria Fritock reported no conflicts of interest. Attestation: Maria Fritock has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Name: Rebecca Y. Klinger, MD.
Contribution: This author helped conduct the study, analyze the data, and write the manuscript.
Conflicts: Rebecca Y. Klinger reported no conflicts of interest. Attestation: Rebecca Y. Klinger has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Name: Roman Sniecinski, MD.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Conflicts: Roman Sniecinski reported no conflicts of interest. Attestation: Roman Sniecinski has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Name: Philip Roman, MD.
Contribution: This author helped conduct the study, analyze the data, and write the manuscript.
Conflicts: Philip Roman reported no conflicts of interest. Attestation: Philip Roman has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Name: Julie Huffmyer, MD.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Conflicts: Julie Huffmyer reported no conflicts of interest. Attestation: Julie Huffmyer has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Name: Michelle Parish, BSN.
Contribution: This author helped conduct the study and write the manuscript.
Conflicts: Michelle Parish reported no conflicts of interest. Attestation: Michelle Parish has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Name: Gayane Yenokyan, PhD.
Contribution: This author helped analyze the data and write the manuscript.
Conflicts: Gayane Yenokyan reported no conflicts of interest. Attestation: Gayane Yenokyan has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files. Name: Charles W. Hogue, MD.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Confiicts: Charles W. Hogue consulted for Covidien and received research funding from Covidien.
Attestation: Charles W. Hogue has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
References
- 1.Zheng F, Sheinberg R, Yee MS, Ono M, Zheng Y, Hogue CW. Cerebral near-infrared spectroscopy monitoring and neurologic outcomes in adult cardiac surgery patients: a systematic review. Anesth Analg. 2013;116:663–76. doi: 10.1213/ANE.0b013e318277a255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steppan J, Hogue CW., Jr Cerebral and tissue oximetry. Best Pract Res Clin Anaesthesiol. 2014;28:429–39. doi: 10.1016/j.bpa.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bickler PE, Feiner JR, Rollins MD. Factors affecting the performance of 5 cerebral oximeters during hypoxia in healthy volunteers. Anesth Analg. 2013;117:813–23. doi: 10.1213/ANE.0b013e318297d763. [DOI] [PubMed] [Google Scholar]
- 4.Heringlake M, Garbers C, Käbler JH, Anderson I, Heinze H, Schön J, Berger KU, Dibbelt L, Sievers HH, Hanke T. Preoperative cerebral oxygen saturation and clinical outcomes in cardiac surgery. Anesthesiology. 2011;114:58–69. doi: 10.1097/ALN.0b013e3181fef34e. [DOI] [PubMed] [Google Scholar]
- 5.Taillefer MC, Denault AY. Cerebral near-infrared spectroscopy in adult heart surgery: systematic review of its clinical efficacy. Can J Anaesth. 2005;52:79–87. doi: 10.1007/BF03018586. [DOI] [PubMed] [Google Scholar]
- 6.Goldman S, Sutter F, Ferdinand F, Trace C. Optimizing intra-operative cerebral oxygen delivery using noninvasive cerebral oximetry decreases the incidence of stroke for cardiac surgical patients. Heart Surg Forum. 2004;7:E376–81. doi: 10.1532/HSF98.20041062. [DOI] [PubMed] [Google Scholar]
- 7.Edmonds HL, Jr, Ganzel BL, Austin EH., III Cerebral oximetry for cardiac and vascular surgery. Semin Cardiothorac Vasc Anesth. 2004;8:147–66. doi: 10.1177/108925320400800208. [DOI] [PubMed] [Google Scholar]
- 8.Edmonds HL., Jr Protective effect of neuromonitoring during cardiac surgery. Ann N Y Acad Sci. 2005;1053:12–9. doi: 10.1196/annals.1344.002. [DOI] [PubMed] [Google Scholar]
- 9.Edmonds HL., Jr Pro: all cardiac surgical patients should have intraoperative cerebral oxygenation monitoring. J Cardiothorac Vasc Anesth. 2006;20:445–9. doi: 10.1053/j.jvca.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103(suppl 1):i3–13. doi: 10.1093/bja/aep299. [DOI] [PubMed] [Google Scholar]
- 11.Murkin JM, Adams SJ, Novick RJ, Quantz M, Bainbridge D, Iglesias I, Cleland A, Schaefer B, Irwin B, Fox S. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth Analg. 2007;104:51–8. doi: 10.1213/01.ane.0000246814.29362.f4. [DOI] [PubMed] [Google Scholar]
- 12.Denault A, Deschamps A, Murkin JM. A proposed algorithm for the intraoperative use of cerebral near-infrared spectroscopy. Semin Cardiothorac Vasc Anesth. 2007;11:274–81. doi: 10.1177/1089253207311685. [DOI] [PubMed] [Google Scholar]
- 13.Slater JP, Guarino T, Stack J, Vinod K, Bustami RT, Brown JM, III, Rodriguez AL, Magovern CJ, Zaubler T, Freundlich K, Parr GV. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg. 2009;87:36–44. doi: 10.1016/j.athoracsur.2008.08.070. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 15.Hogue CW, Jr, Palin CA, Arrowsmith JE. Cardiopulmonary bypass management and neurologic outcomes: an evidence-based appraisal of current practices. Anesth Analg. 2006;103:21–37. doi: 10.1213/01.ANE.0000220035.82989.79. [DOI] [PubMed] [Google Scholar]
- 16.Speir AM, Kasirajan V, Barnett SD, Fonner E., Jr Additive costs of postoperative complications for isolated coronary artery bypass grafting patients in Virginia. Ann Thorac Surg. 2009;88:40–5. doi: 10.1016/j.athoracsur.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 17.Tarakji KG, Sabik JF, III, Bhudia SK, Batizy LH, Blackstone EH. Temporal onset, risk factors, and outcomes associated with stroke after coronary artery bypass grafting. JAMA. 2011;305:381–90. doi: 10.1001/jama.2011.37. [DOI] [PubMed] [Google Scholar]
- 18.Murkin JM, Martzke JS, Buchan AM, Bentley C, Wong CJ. A randomized study of the influence of perfusion technique and pH management strategy in 316 patients undergoing coronary artery bypass surgery. I. Mortality and cardiovascular morbidity. J Thorac Cardiovasc Surg. 1995;110:340–8. doi: 10.1016/S0022-5223(95)70229-6. [DOI] [PubMed] [Google Scholar]
- 19.Germon TJ, Young AE, Manara AR, Nelson RJ. Extracerebral absorption of near infrared light influences the detection of increased cerebral oxygenation monitored by near infrared spectroscopy. J Neurol Neurosurg Psychiatry. 1995;58:477–9. doi: 10.1136/jnnp.58.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davie SN, Grocott HP. Impact of extracranial contamination on regional cerebral oxygen saturation: a comparison of three cerebral oximetry technologies. Anesthesiology. 2012;116:834–40. doi: 10.1097/ALN.0b013e31824c00d7. [DOI] [PubMed] [Google Scholar]
- 21.Al-Rawi PG, Smielewski P, Kirkpatrick PJ. Evaluation of a near-infrared spectrometer (NIRO 300) for the detection of intracranial oxygenation changes in the adult head. Stroke. 2001;32:2492–500. doi: 10.1161/hs1101.098356. [DOI] [PubMed] [Google Scholar]