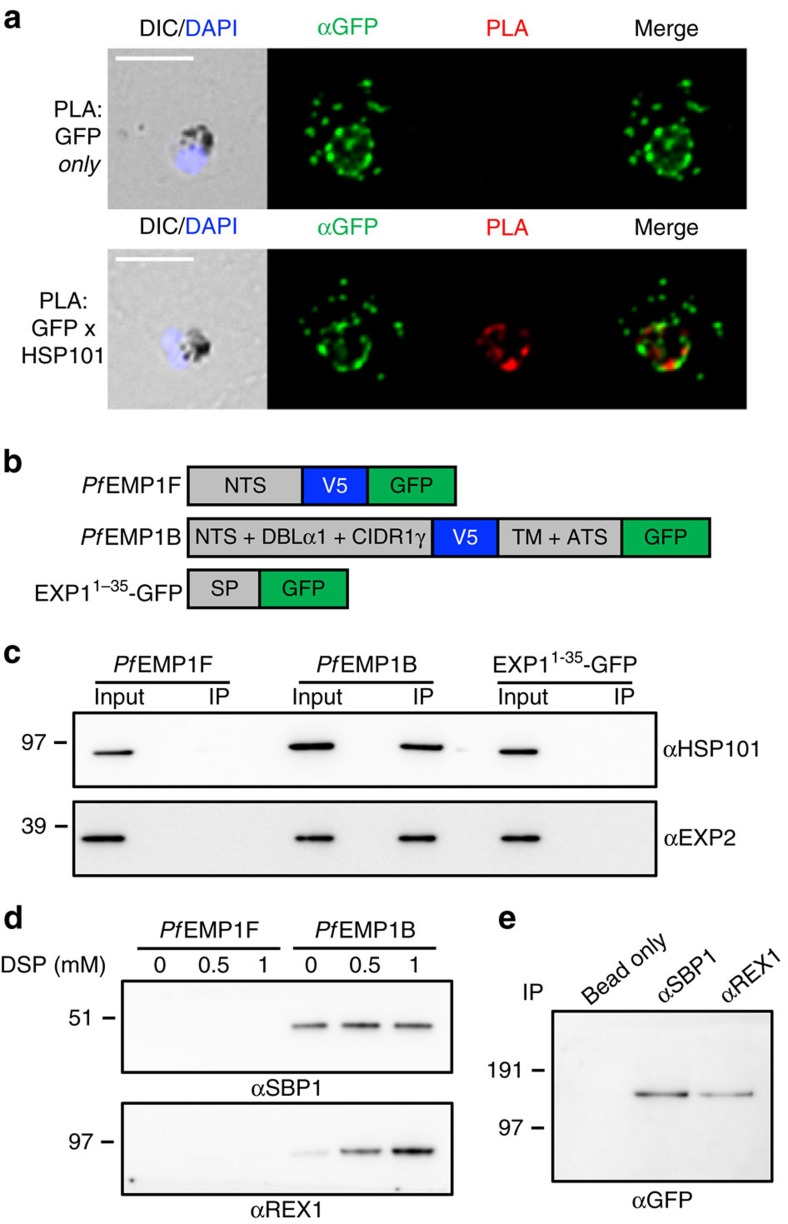
Figure 2. PfEMP1B interacts with components of PTEX and the Maurer’s clefts.
(a) Proximity ligation analysis (PLA) of PfEMP1B-infected RBCs labelled with antisera recognizing GFP alone (top) or GFP and HSP101 (bottom). A PLA signal is only produced if the primary epitopes are ≤40 nm apart. DIC and DAPI (blue), anti-GFP (green) and PLA (red) are shown. Scale bars, 5 μm. (b) Schematic of GFP-fusion proteins used in immunoprecipitation (IP) experiments. PfEMP1F contains the N-terminal sequence (NTS) from ITvar3 followed by a V5 epitope tag and GFP and is located in the parasite cytoplasm as previously described16. PfEMP1B is as described in Fig. 1a. EXP11-35-GFP contains the signal peptide (SP) from EXP1 followed by GFP and localizes to the PV as previously described47. (c) Immunoprecipitation of PfEMP1F, PfEMP1B and EXP11-35-GFP transfectants using GFP-Trap. Total protein (input) and eluate (IP) were prepared for western blot and probed with anti-HSP101 and anti-EXP2 antisera. (d) Immunoprecipitation of PfEMP1F and PfEMP1B parasite lysates crosslinked using increasing concentrations of DSP. Western blots were probed with anti-SBP1 and anti-REX1 antisera. (e) Reverse IP on crosslinked PfEMP1B parasite lysate using SBP1 and REX1 antisera. Western blots were probed with anti-GFP antisera. Precipitation of agarose beads with no antiserum was used as a control. (c–e) Molecular masses are shown in kDa. Uncropped western blots are shown in Supplementary Fig. 12.