For the past three decades, nearly half of all new lung cancer cases each year were metastatic at diagnosis (e.g., 57% of incident lung cancers were stage IV between 2006 and 2013).1 Stage at diagnosis has consistently weighed heavily toward advanced disease, in part, because chest x-ray and sputum cytology are ineffective screening modalities.2 In 2011, the National Lung Screening Trial concluded that adherence to a protocol of annual computed tomography (CT) lung cancer screening and follow-up reduced lung cancer mortality by 20% more compared with chest x-ray screening.3
A recent population-based study identified a key problem in achieving the promise of CT screening observed in the trial setting to everyday practice.4 The prevalence of CT screening within the past year among eligible adults in the United States has remained virtually stagnant over time: 3.3% in 2010 and 3.9% in 2015.4 We identify a second key problem: cigarette smokers continue to receive chest x-ray screening rather than CT screening, despite clinical recommendations against the former and in favor of the latter.
CONTEMPORARY LUNG CANCER SCREENING RATES
We used data from the 2015 National Health Interview Survey, Cancer Control Supplement (NHIS CCS) and calculated across levels of risk of lung cancer the prevalence of (1) chest x-ray lung cancer screening within the past year (“Were any of the chest x-rays you had in the last 12 months done to check for lung cancer rather than for some other reason?”), (2) CT lung cancer screening within the past year (“When did you have your most recent CT or CAT scan of your chest area to check or screen for lung cancer?”), and (3) intention to receive CT lung cancer screening within the next year (“less than a year from now” or “one year from now” to survey question “When do you expect to have your next CT scan of your chest area to check or screen for lung cancer?”). We estimated the risk of lung cancer by applying the Tammemägi et al.5 risk estimation model that incorporates sociodemographic and smoking-related risk factors, comorbidities, and cancer history among the 13 201 ever cigarette smokers sampled in the 2015 NHIS CCS. We also calculated the prevalence of these three outcomes among adults eligible for CT lung cancer screening based on Centers for Medicare and Medicaid Services (CMS) criteria.6
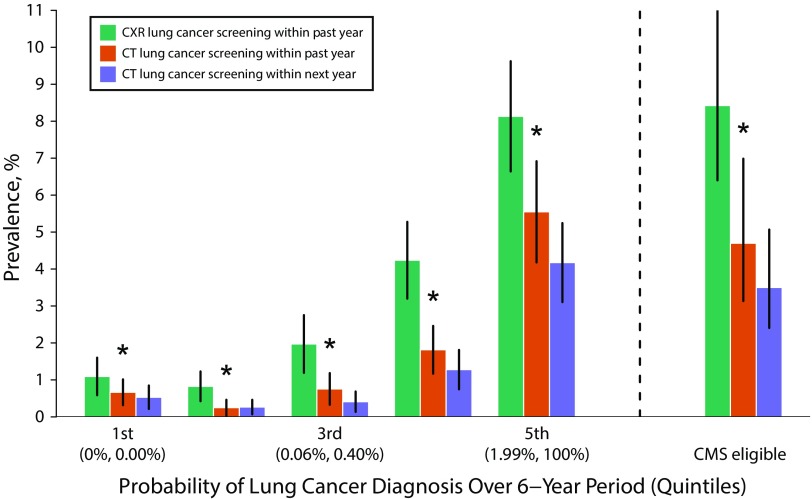
We found several concerning results. First, within the past year, the prevalence of chest x-ray screening was significantly higher than the corresponding prevalence of CT screening across nearly all risk quintiles and among CMS-eligible adults (Figure 1). For example, among ever smokers in the highest quintile of risk, the prevalence of chest x-ray screening within the past year was 8.1% (95% confidence interval [CI] = 6.6%, 9.6%) compared with 5.5% (95% CI = 4.2%, 6.9%) for CT screening within the past year. Second, only six of every 10 (59.7%) adult smokers who received CT screening within the past year intended to receive CT screening within the next year. Third, adult smokers were more—not less—likely to have received chest x-ray screening within the past year with greater health care use: 0.8% (95% CI = 0.2%, 1.4%) for adults with no physician visits in the past 12 months, 1.8% (95% CI = 1.3%, 2.3%) for adults with one to two visits, and 5.2% (95% CI = 4.4%, 6.0%) for adults with three or more visits.
FIGURE 1—
Prevalence of Computed Tomography (CT) Lung Cancer Screening Within the Past Year, Chest X-Ray (CXR) Lung Cancer Screening Within the Past Year, and Intention to Receive CT Lung Cancer Screening Within the Next Year, by Risk of Lung Cancer Among Ever Cigarette Smokers and Centers for Medicare & Medicaid Services (CMS) Eligibility: 2015 National Health Interview Survey, Cancer Control Supplement, United States
Note. 95% confidence intervals shown as vertical lines. An asterisk represents a statistically significant difference between CT screening within the past year and CXR screening within the past year accounting for multiple comparisons.
Encouragingly, we found that CT screening within the past year and intention to receive CT screening in the next year both increased for adult smokers with several key clinical risk factors, including chronic obstructive pulmonary disease (COPD), personal history of cancer, and family history of lung cancer. For example, the odds of CT screening within the past year were higher for adult smokers with COPD than for those without COPD (adjusted odds ratio [AOR] = 2.5; 95% CI = 1.5, 4.1; Table A, available as a supplement to the online version of this article at http://www.ajph.org).
Screening Practices vs Clinical Evidence
The prevalence of chest x-ray screening may be higher than that of CT screening because of gaps in physicians’ knowledge about screening guidelines and the potential benefits of screening.7–9 Physicians may not be abreast on the latest guidelines about lung cancer screening because of the sheer volume of clinical practice guidelines. Moreover, physicians may not view clinical practice guidelines as trustworthy because the vast majority of guidelines fail to adhere to most standards set forth by the Institute of Medicine.10
Physicians may continue to use chest x-rays to screen for lung cancer because of the decades-long practice of screening for cancer with this modality.11 Physicians also may continue this practice because of the legacy of chest x-ray screening from the Mayo Lung Project trial (1971–1983) and the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial (1993–2001). The Mayo trial found no significant short- or long-term reduction in the lung cancer mortality rate between the intervention (chest x-ray and sputum cytology every four months for six years) and the usual-care arm (recommendation at beginning of trial to obtain an annual chest x-ray and sputum cytology).12 Similarly, the PLCO trial found that chest x-ray screening did not reduce lung cancer mortality between the intervention (annual chest x-ray for four years) and the usual-care arms.2 However, physicians still may believe that chest x-ray affords their patients some clinical benefit because the Mayo trial found that participants in the intervention arm experienced longer survival, and both trials found an increased rate of earlier detection. A trial of primary care physicians found a systematic bias wherein they mistakenly interpreted improved survival and earlier detection as sufficient evidence to conclude that a cancer screening test was effective.13
A commission bias, pervasive in the culture of medicine, also may lead physicians to screen with chest x-ray—despite its lack of proven efficacy—rather than to do nothing at all, especially for patients at high risk for lung cancer.14 For example, we found that the odds of chest x-ray screening within the past year were higher for adults with COPD than for those without COPD (AOR = 2.2; 95% CI = 1.5, 3.3; Appendix A).
Obstacles and Opportunities
We can simultaneously increase the rate of CT screening and decrease the rate of chest x-ray screening for lung cancer through several health care system–level changes and patient engagement. Currently, unlike breast, cervical, and colorectal cancer screening, CT screening for lung cancer is not a CMS quality measure or a Healthcare Effectiveness Data and Information Set measure. Adding CT lung cancer screening to these quality measures may incentivize health care systems to offer CT screening to patients at high risk for lung cancer. Electronic health records also could report smoking history in pack-years, which would enable clinicians to focus time- and resource-intensive screening for patients most at risk.15 In addition, medical societies and lung cancer advocacy groups should develop educational programs for health care providers around the evidence base, clinical guidelines, potential harms (e.g., false-positive CT screening results), cost, and reimbursement requirements of CT screening.
System-level improvements alone may not increase CT screening. Smokers may not seek screening because they underestimate their risk of lung cancer and do not necessarily believe that earlier detection of lung cancer improves survival.16 Public health awareness campaigns could reduce misinformation, misperception, and reluctance about CT screening. Additionally, telephone-based support delivered by trained staff increases the rate of screening for breast, cervical, and colorectal cancer and is cost-effective. A similar program could help adult smokers overcome barriers to receive CT screening, and ensure adherence to an annual screening regimen and timely follow-up visits for positive screening results.
ACKNOWLEDGMENTS
Financial support for this study was provided by the National Institutes of Health (R21CA197912 to S. Soneji) and the American Lung Association (Lung Cancer Discovery Award to S. Soneji).
We acknowledge Shila Soneji for her critical review and comments.
HUMAN PARTICIPANT PROTECTION
This study involved only analysis of a public data set (the 2015 National Health Interview Survey [NHIS]) and did not meet the regulatory definition of human participant research. This study did not involve merging the NHIS data with any other data sets in such a way that individuals might be identified. This study did not enhance the public data set with identifiable or potentially identifiable data.
REFERENCES
- 1.Howlader N, Noone A, Krapcho M . SEER Cancer Statistics Review, 1975-2012. Bethesda, MD: National Cancer Institute; 2015. [Google Scholar]
- 2.Oken MM, Hocking WG, Kvale PA et al. Screening by chest radiograph and lung cancer mortality: the Prostate, Lung, Colorectal, and Ovarian (PLCO) randomized trial. JAMA. 2011;306(17):1865–1873. doi: 10.1001/jama.2011.1591. [DOI] [PubMed] [Google Scholar]
- 3.National Lung Screening Trial Research Team. Aberle DR, Adams AM et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Fedewa SA. Lung cancer screening with low-dose computed tomography in the United States—2010 to 2015. JAMA Oncol. 2017 doi: 10.1001/jamaoncol.2016.6416. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tammemägi MC, Katki HA, Hocking WG et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368(8):728–736. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Syrek Jensen T, Chin J, Ashby L, Hermansen J, Dolph Hutter J. Decision Memo for Screening for Lung Cancer With Low Dose Computed Tomography (LDCT) (CAG-00439N) Baltimore, MD: Centers for Medicare & Medicaid Services; 2015. Available at: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274. Accessed April 24, 2017. [Google Scholar]
- 7.Klabunde CN, Marcus PM, Silvestri GA et al. U.S. primary care physicians’ lung cancer screening beliefs and recommendations. Am J Prev Med. 2010;39(5):411–420. doi: 10.1016/j.amepre.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klabunde CN, Marcus PM, Han PKJ et al. Lung cancer screening practices of primary care physicians: results from a national survey. Ann Fam Med. 2012;10(2):102–110. doi: 10.1370/afm.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ersek JL, Eberth JM, McDonnell KK et al. Knowledge of, attitudes toward, and use of low-dose computed tomography for lung cancer screening among family physicians. Cancer. 2016;122(15):2324–2331. doi: 10.1002/cncr.29944. [DOI] [PubMed] [Google Scholar]
- 10.Reames BN, Krell RW, Ponto SN, Wong SL. Critical evaluation of oncology clinical practice guidelines. J Clin Oncol. 2013;31(20):2563–2568. doi: 10.1200/JCO.2012.46.8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mauri D, Kamposioras K, Proiskos A et al. Old habits die hard: chest radiography for screening purposes in primary care. Am J Manag Care. 2006;12(11):650–656. [PubMed] [Google Scholar]
- 12.Marcus PM, Bergstralh EJ, Fagerstrom RM et al. Lung cancer mortality in the Mayo Lung Project: impact of extended follow-up. J Natl Cancer Inst. 2000;92(16):1308–1316. doi: 10.1093/jnci/92.16.1308. [DOI] [PubMed] [Google Scholar]
- 13.Wegwarth O, Schwartz LM, Woloshin S, Gaissmaier W, Gigerenzer G. Do physicians understand cancer screening statistics? A national survey of primary care physicians in the United States. Ann Intern Med. 2012;156(5):340–349. doi: 10.7326/0003-4819-156-5-201203060-00005. [DOI] [PubMed] [Google Scholar]
- 14.Groopman J. How Doctors Think. Boston, MA: Houghton Mifflin; 2007. [Google Scholar]
- 15.National Academies of Sciences, Engineering, and Medicine. Implementation of Lung Cancer Screening: Proceedings of a Workshop. Washington, DC: National Academies Press; 2016. [PubMed] [Google Scholar]
- 16.Silvestri GA, Nietert PJ, Zoller J, Carter C, Bradford D. Attitudes towards screening for lung cancer among smokers and their non-smoking counterparts. Thorax. 2007;62(2):126–130. doi: 10.1136/thx.2005.056036. [DOI] [PMC free article] [PubMed] [Google Scholar]