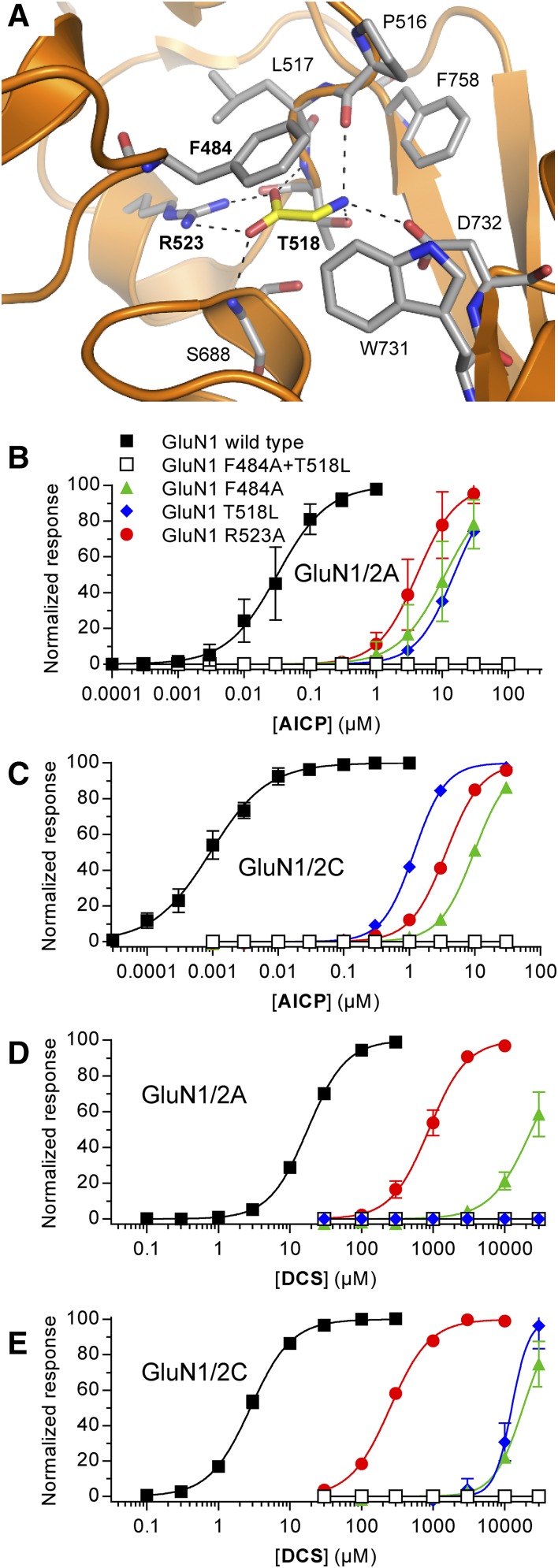
Fig. 3.
Activity of AICP and DCS at NMDA receptors with mutations in the glycine binding site. (A) Structure of glycine bound in the agonist binding site of the GluN1 subunit (PDB ID 5I57; Yi et al., 2016). Dashed lines indicate polar interactions between glycine (yellow carbon) and GluN1 residues (gray carbon). Mutated residues are indicated in bold text. (B–E) Concentration–response data for AICP (B and C) and DCS (D and E) at rat GluN1/2A and GluN1/2C receptors with mutations in the glycine binding site measured using two-electrode voltage-clamp recordings. The responses are normalized to the fitted maximal response. Data are mean ± S.D. from 3 to 29 oocytes, and the EC50 values are listed in Supplemental Table 2.