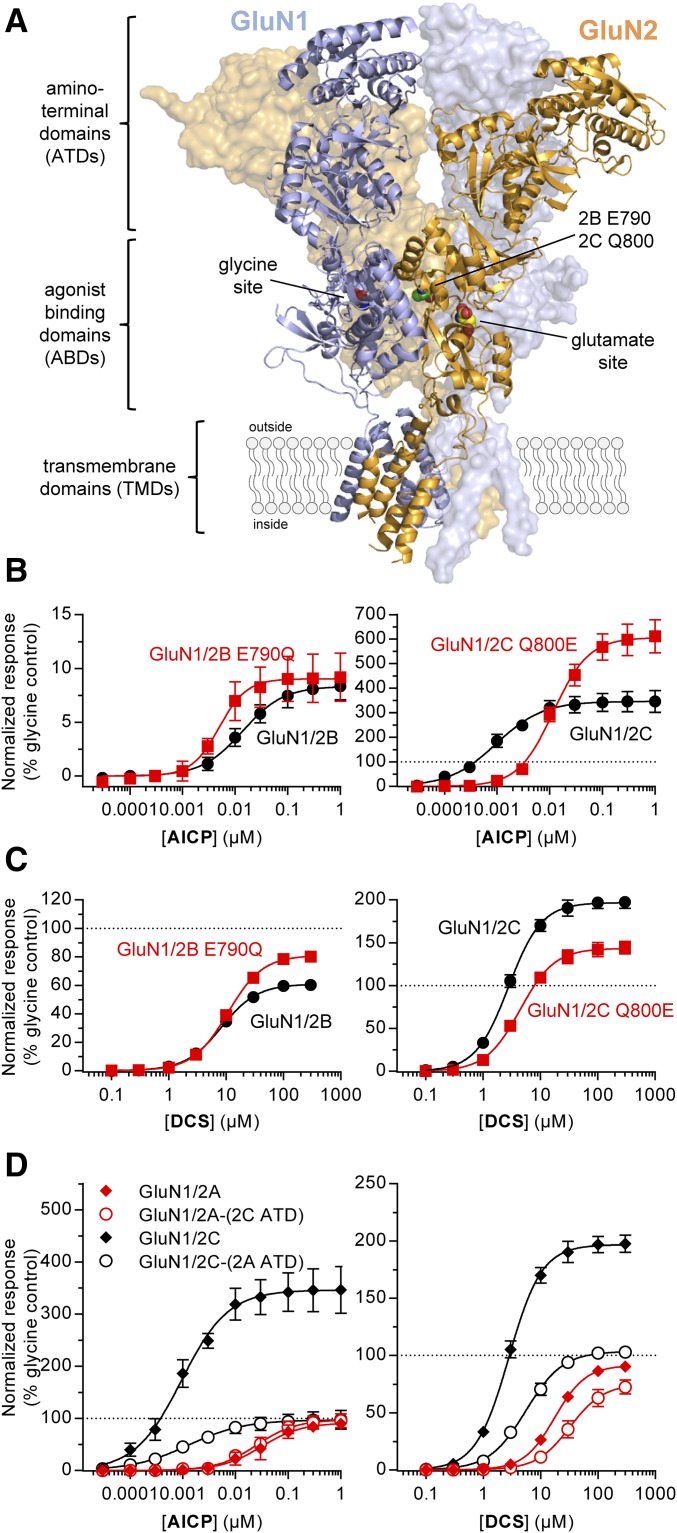
Fig. 5.
Relative agonist efficacy of AICP is controlled by residues in multiple receptor domains. (A) NMDA receptor structure (PDB ID 4PE5; Karakas and Furukawa, 2014) illustrating the subunit arrangement and the layered domain organization composed of the transmembrane domains (TMDs) and two extracellular layers formed by the agonist binding domains (ABDs) and the ATDs. Agonist binding sites for glycine in GluN1 and glutamate in GluN2 subunits as well as the known positions of homologous residues E790 in GluN2B and Q800 in GluN2C are highlighted. (B, C) Concentration–response data for (B) AICP and (C) DCS measured using two-electrode voltage-clamp recordings at rat wild-type and mutant GluN1/2B and GluN1/2C receptors. Data are mean ± S.D. from 8 to 18 oocytes. (D) Concentration–response data for AICP and DCS measured using two-electrode voltage-clamp recordings at rat GluN1/2A and GluN1/2C receptors and receptors with interchanged ATDs [GluN1/2A-(2C ATD) and GluN1/2C-(2A ATD)]. Data are mean ± S.D. from 6 to 29 oocytes. The EC50 values and relative agonist efficacies (i.e., maximal responses relative to glycine) are listed in Supplemental Table 4.