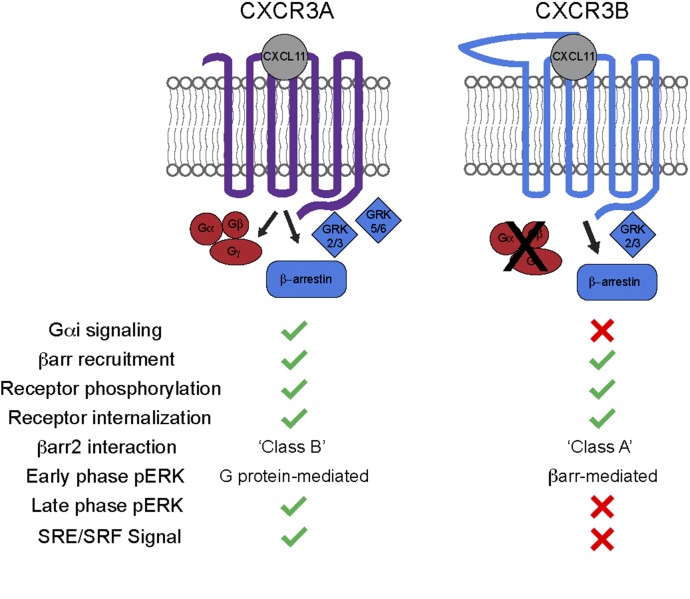
Fig. 12.
Summary of observed CXCR3 isoform signaling. Both CXCR3A and CXCR3B recruited β-arrestin2, became phosphorylated, and internalized in response to CXCL11. In contrast, only CXCR3A was observed to signal through Gαi since CXCR3B-transfected cells did not produce appreciable signal in either Gαi or Gαs assays. In further signaling divergence, CXCR3A and CXCR3B show distinct patterns of downstream signaling, with CXCR3A observed to display a stable class B interaction with β-arrestin2 in contrast to CXCR3B, which displayed a transient class A interaction in confocal recruitment assays. GRK2/3 siRNA knockdown attenuated βarr2 recruitment to both receptor isoforms; however, only GRK5/6 knockdown was observed to attenuate βarr2 recruitment to CXCR3A. Only CXCR3A was observed to show significant late phase (1 hour) pERK activity, and overexpression rescue of β-arrestin attenuated early phase pERK activity mediated by CXCR3A while enhanced pERK activity was mediated by CXCR3B. SRE and SRF transcriptional reporter activity was only observed downstream from CXCR3A.