Figure 2.
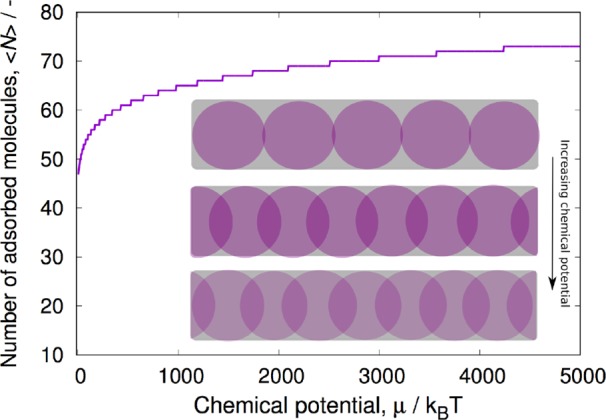
Adsorption isotherm of WCA particles in a cylindrical pore. Inset: schematic representation of adsorbed states. A pore length of 50 dimensionless units was used. It is always possible to adsorb a new particle if the chemical potential is high enough. This is visible as the “staircase” effect, where the “steps” become longer and longer, i.e., it takes progressively more pressure to push another particle in. Every time a new particle is adsorbed, the already adsorbed particles have to redistribute equidistantly to reduce the repulsive interactions. This requires the simultaneous collective motion of all adsorbed particles.
