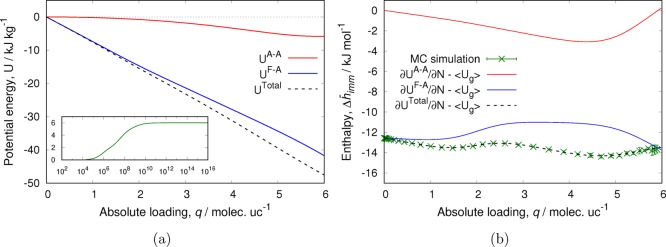
Figure 9.
Adsorption of CH4 in CHA-type zeolite at 300 K: (a) total, framework–adsorbate, and adsorbate–adsorbate energies as a function of loading obtained from NVT MC simulations (inset: isotherm, loading in molecules per unit cell as a function of fugacity in Pa) and (b) enthalpy of adsorption computed from the fluctuation formula using grand-canonical MC simulations and derivatives of the energies of (a) with respect to loading. Using this energy decomposition, the enthalpy can be decomposed in the contribution from the framework–adsorbate and adsorbate–adsorbate interactions.