Abstract
Objective:
Diabetes mellitus, smoking, dyslipidemia, and obesity play an important role in the etiology of erectile dysfunction, particularly in cases with vascular insufficiency. These risk factors also target the lungs due to their systemic effects.
Materials and Methods:
Patients with penile vascular insufficiency determined at Doppler ultrasonography and undergoing thoracic computerized tomography for various reasons were included in this study. A history of acute thoracic trauma, pneumonic consolidation, or pelvic surgery and trauma were regarded as exclusion criteria.
Results:
Thirty-seven male patients with identified vascular insufficiency (age 54.48 ± 13.62 years) were enrolled. Mass lesions with a malignant morphology were present in two patients. The most common mediastinal/vascular pathology was atherosclerosis, while the most common parenchymal lesion was emphysematous aeration. Other findings included parenchymal fibrotic bands, atelectasis, interstitial thickening, bronchiectasis, air trapping, aortic aneurysm, a dilated pulmonary artery, hiatal hernia, and pericardial effusion.
Conclusion:
Erectile dysfunction may be an early sign of cardiovascular diseases. Care must be taken in terms of existing or potential pulmonary pathologies in these patients due to their sharing common risk factors with systemic effects.
KEYWORDS: Erectile dysfunction, lung, penile vascular insufficiency

INTRODUCTION
Penile erection involves a coordinated organization of neural and vascular functions. The formation of cyclic guanylate monophosphate and cyclic adenylate monophosphate increases as a result of nitric oxide (NO) secretion.[1] Phosphorylation of the potassium and calcium channels thus occurs, and erection takes places as cavernousum fill with blood.[1,2] The peripheral veins are contracted, and the level of blood returning is reduced in order for the erection to be maintained.[2]
Although erectile dysfunction is a benign condition, it impairs the quality of life of the patient and his partner. The primary risk factors for erectile dysfunction include hormonal disorders, neurological and cardiovascular diseases, dyslipidemia, diabetes mellitus, hypertension, low physical activity, smoking, and obesity.[3] Smoking, diabetes, dyslipidemia, and obesity are particularly dominant risk factors in patients with erectile dysfunction with vascular insufficiency.[2] These are also leading predisposing factors for respiratory system diseases and dysfunction. Smoking is known to be associated with chronic obstructive pulmonary disease (COPD), lung cancer, idiopathic pulmonary fibrosis (IPF), respiratory bronchiolitis, desquamative interstitial pneumonia, and pulmonary Langerhans cell histiocytosis.[4] Similarly, recent studies have shown that diabetes affects the lung as a new target organ and causes respiratory dysfunction. Various factors have been implicated in pulmonary dysfunction in diabetic patients, such as microangiopathy, neuropathy, an increased incidence of infection, nonenzymatic glycosylation, abnormal pulmonary elasticity, and decreased dynamic compliance.[5] Overloading in the respiratory muscles reducing compliance and increasing total respiratory system resistance are examples of changes occurring in the lungs in obese patients.[6] The purpose of this study was to evaluate radiological findings determined at thoracic computed tomography (CT) performed for various reasons on patients with erectile dysfunction.
MATERIALS AND METHODS
Study design and ethics
Patients presenting to the urology clinic due to erectile dysfunction (in 2011–2016) and with vascular insufficiency identified at penile Doppler ultrasonography were evaluated retrospectively. The radiology archive was searched, and patients undergoing thoracic CT for reasons other than thoracic trauma were included in the study. The exclusion criteria were acute thoracic trauma, active pneumonic consolidation, pelvic trauma, and a history of pelvic surgery. Ethical approval was granted by the faculty of medicine. Patients' cholesterol, triglyceride, and blood sugar levels and International Index of Erectile Function (IIEF) scores at presentation were documented from their files.
Imaging techniques
Doppler ultrasonography was performed using standard procedures on Toshiba Aplio XG and General Electric Logiq 9 sonography devices (7.5 MHz and 10 MHz linear transducers, respectively). Anatomical structures and presence of fibrous plaque were investigated with gray-scale examination in the supine position, and vascular structures were investigated with Doppler sonography. Papaverine was injected into the cavernous body with an insulin injector, and spectral wave analyses were performed at 5, 10, and 20 min. The Doppler angle was kept as close to 60° as possible. An erection angle of 90° or above, a peak systolic velocity >35 cm/s and an end-diastolic velocity < 5 cm/s were regarded as normal. Thoracic CT images (Toshiba, Asteion TSX-021B, 4 detector tomography device, 120 kV, 150 mAs, 5 mm section thickness) obtained with a routine protocol and CT images were evaluated by a radiologist.
Statistical analyses
Statistical analysis was performed using SPSS 19.0 (SPSS Inc, Chicago, IL, USA) for Windows software. The Chi-square test was used for quantitative data, and Student's t-test was used to analyze qualitative data.
RESULTS
Thirty-seven male patients (aged 54.48 ± 13.62 years) with penile vascular insufficiency determined at Doppler ultrasonography and whose thoracic CT images were available in the radiology archive were included in the study. Venous insufficiency was determined with Doppler ultrasonography in 20 cases (54.1%) and arterial insufficiency in 17 (45.9%). Chronic smoking status was determined in 26 patients (70.3%), a history of diabetes in 17 (45.9%), and a combination of diabetes and smoking in 11 (29.72%). Neither risk factor was present in four patients (10.8%).
The evaluation of mediastinal and pleural structures revealed atherosclerosis in 23 cases (62.2%), mediastinal lymph node with a short axis >6 mm in 14 (37.8%), and thickening of pleural and extrapleural fat tissue in six patients (16.2%). Other mediastinal and pleural findings were aortic aneurysm, pleural effusion, and hiatal hernia. Pericardial effusion accompanying pleural effusion was present in one case [Figure 1]. The distribution of mediastinal and pleural radiological findings is shown in Table 1.
Figure 1.
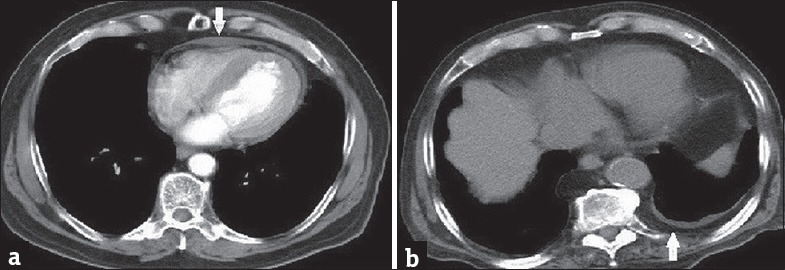
A 68-year-old patient presenting with dyspnea (a) Contrast-enhanced computed tomography image with mediastinal window shows pericardial effusion. A 59-year-old patient presenting with dyspnea (b) Computed tomography image with mediastinal window shows extrapleural fat tissue/pleural thickening.
Table 1.
Mediastinal radiological findings

The most common findings at pulmonary parenchymal evaluation were emphysematous aeration and parenchymal fibrotic bands (48.6% and 40.5%, respectively). Other parenchymal changes were subsegmental atelectasis, interstitial thickening, bronchiectasis, air trapping and ventilation inhomogeneity, focal ground-glass density, and mass formation [Figures 2 and 3]. Changes observed in the pulmonary parenchyma are shown in Table 2.
Figure 2.
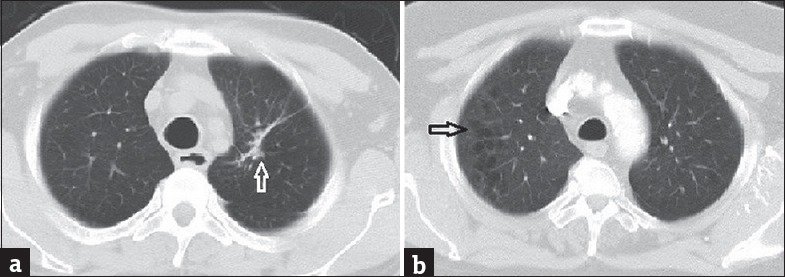
Axial computed tomography images with lung window of two patients (age 68 and 61 years, respectively) presenting with shortness of breath show parenchymal fibrotic band (a) and centrilobular pulmonary emphysema (b)
Figure 3.
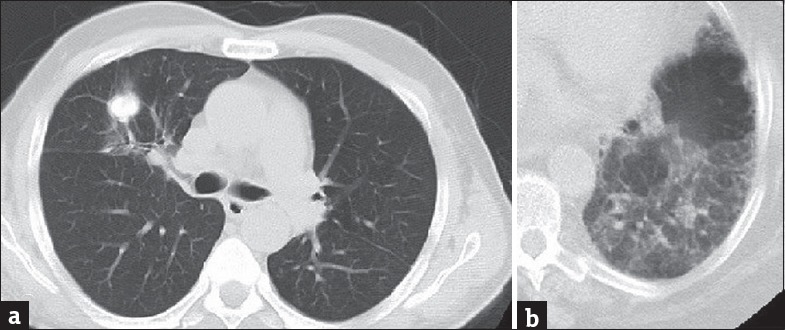
A 68-year-old patient presenting with chronic cough (a) Axial computed tomography image shows pulmonary mass with irregular margins. Contrast-enhanced axial computed tomography image (b) with lung window shows air trapping in a 59-year-old patient presenting with shortness of breath
Table 2.
Radiological findings observed in the parenchyma

DISCUSSION
In addition to hemodynamic events, erection also depends on psychogenic and endocrinological events.[1] Smoking, diabetes, and obesity lead to the development of erectile dysfunction by causing vascular insufficiency.[2] These are also the most important risk factors for cardiovascular and respiratory system pathologies. Ehrlich et al., assessed 77,637 patients with diabetes and 1,733,591 nondiabetic patients in terms of pulmonary pathologies. They observed a high incidence of several pathologies, such as pneumonia, asthma, COPD, and pulmonary fibrosis, in diabetic patients but reported that this was not linked to lung cancer.[7] The most important etiological factor in lung cancer is smoking.[8] Mass lesions with a malignant morphology were present in two patients. Moreover, the diagnosis was confirmed histopathologically in one. A mass lesion with irregular contours and multiple pulmonary parenchymal nodules were present in the second patient. However, the histopathological results were unavailable since the patient wished to continue treatment at an external center. In this study, in addition to pathologies induced by chronic inflammation in patients with erectile dysfunction, such as emphysema, chronic bronchitis, and bronchiectasis, we also evaluated aortic, coronary, and pulmonary vascular structures. We also investigated elementary lesions such as fibrotic parenchymal bands, atelectasis, and air trapping in these patients.
Emphysematous aeration was present in 18 cases in our study and focal hypoattenuation compatible with air trapping in five. Increased inflammation, oxidative stress, and protease/antiprotease imbalance also play a fundamental role in COPD. Proteases involved in pulmonary parenchymal destruction are neutrophil elastase, proteinase 3, cathepsin G, and matrix metalloproteinases.[9] Proteolysis, fibrosis, and structural changes in the airways/parenchyma may occur as a result of increased inflammatory response in the airways and pulmonary parenchyma due to smoking. One noteworthy point in this study is that emphysema was determined in 10 of the 11 diabetic patients who smoked. At least, one risk factor was present in another six patients with emphysema, while only one patient did not smoke and did not have diabetes. Similarly, both risk factors were present in four of the five patients with air trapping. Another finding of this study is the greater duration of erectile dysfunction in cases with emphysema compared to those without emphysema (5.22 ± 4.65 and 2.00 ± 1.85 years, respectively; P = 0.008).
A decrease in glutathione peroxidase activity, endothelial dysfunction caused by NO, an increase in heparan sulfate levels in the basal membrane and accumulation of collagen and elastin in the alveolar walls may occur in patients with diabetes.[10] Experimental studies have shown that effects in the alveoli are responsible for emphysema and that collagen and elastin accumulation is responsible for changes in elasticity.[11] Our findings support the idea that diabetes and smoking together exhibit a synergistic effect in the development of emphysema.[4,5]
In addition to regulating vascular tonus and vasodilation, NO also protects against vascular damage, inflammation, and thrombosis. In addition, it restricts leukocyte adhesion to the endothelium, smooth muscle cell proliferation and platelet aggregation. Major risk factors for atherosclerosis such as hypertension, diabetes, dyslipidemia, and smoking compromise its protective functions.[12] Atherosclerotic changes were present in 23 patients in our study, aortic aneurysm in five, and a dilated pulmonary artery in three. Atherosclerosis was detected in all patients with aortic aneurysm.
The other elementary lesions commonly encountered in our study were parenchymal bands or subsegmental atelectasis. Parenchymal bands are a marker of parenchymal fibrosis. They indicate interstitial involvement in the initial period, and their prevalence is higher among smokers.[13] Moreira et al., reported the prevalence of parenchymal fibrotic bands of 78.6% in patients with COPD and of 22.6% in healthy control group.[14] The prevalence in our study was 40.5%. We think that the difference may be attributed to the mean age in that study being 64.8 years and to the entire patient group having COPD.
Obstruction in the small airways may occur in COPD. Hypertrophy and inflammatory cell infiltration in the bronchial glands, ciliary dysfunction, an increase in smooth muscle and connective tissue and mucus hypersecretion are implicated in the pathophysiology.[14,15] Similar peripheral airway obstructions can be seen in diabetic patients. Connective tissue changes secondary to nonenzymatic glucolization, capacity loss in the respiratory muscles, bronchoconstriction secondary to autonomous neuropathy, increased airway sensitivity, and decreased pulmonary elastic recoil cause peripheral airway obstruction in these patients.[16] Moreira et al., reported that linear atelectasis also increased in COPD, with the prevalence of 28.6%.[14] We determined atelectasis in seven patients (18.9%) in this study of subjects with erectile dysfunction. At least one of diabetes mellitus or smoking was present in six of the seven patients with atelectasis.
Pulmonary infections are an important cause of changes such as parenchymal fibrotic bands, atelectasis, and bronchiectasis. Impaired polymorphonuclear leukocyte functions such as decreased phagocytic activity, chemotaxis, and adherence are an important cause of an increasing disposition to infections in these patients.[17] The incidences of tuberculosis, influenza virus, mucormycosis, and aspiration pneumonia particularly increase. Prognosis of pulmonary infections is also poorer.[17,18] In agreement with the literature, two of our patients had a previous history of tuberculosis.
One experimental study in animals with diabetes mellitus showed an accumulation of collagen and elastin in the basal lamina and interstitium involvement.[19] Respiratory bronchiolitis, desquamative interstitial pneumonia, and pulmonary Langerhans cell histiocytosis are interstitial lung diseases associated with smoking. Acute eosinophilic pneumonia and IPF are also precipitated by smoking.[20] Interstitial thickening was present in two of our patients. Since the high-resolution CT protocol was not available in our study, our interstitium evaluation was not optimal. In addition to the effect on the lungs of factors in the etiology of erectile dysfunction, the prevalence of erectile dysfunction also increases in chronic pulmonary diseases. Reduced exercise tolerance in chronic diseases such as chronic lung disease, obesity, and diabetes and fear of dyspnea trigger erectile dysfunction. Pulmonary rehabilitation programs can increase patients' daily activity capacities.[21]
CONCLUSION
When psychological and hormonal causes are excluded, factors involved in the etiology of erectile dysfunction also cause morphological and functional impairments in the lungs. Care must be taken in terms of pulmonary pathologies already present, or that may occur in the near future in these patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2017/7/1/25/209093
REFERENCES
- 1.Angulo J, Cuevas P, Gabancho S, Gonzalez-Corrochano R, Videla S, Saenz de Tejada I. Enhancement of both EDHF and NO/cGMP pathways is necessary to reverse erectile dysfunction in diabetic rats. J Sex Med. 2005;2:341–6. doi: 10.1111/j.1743-6109.2005.20348.x. [DOI] [PubMed] [Google Scholar]
- 2.Lue TF. Erectile dysfunction. N Engl J Med. 2000;342:1802–13. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- 3.Braun M, Wassmer G, Klotz T, Reifenrath B, Mathers M, Engelmann U. Epidemiology of erectile dysfunction: Results of the 'Cologne Male Survey'. Int J Impot Res. 2000;12:305–11. doi: 10.1038/sj.ijir.3900622. [DOI] [PubMed] [Google Scholar]
- 4.Caminati A, Cavazza A, Sverzellati N, Harari S. An integrated approach in the diagnosis of smoking-related interstitial lung diseases. Eur Respir Rev. 2012;21:207–17. doi: 10.1183/09059180.00003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitocco D, Fuso L, Conte EG, Zaccardi F, Condoluci C, Scavone G, et al. The diabetic lung – A new target organ? Rev Diabet Stud. 2012;9:23–35. doi: 10.1900/RDS.2012.9.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steier J, Lunt A, Hart N, Polkey MI, Moxham J. Observational study of the effect of obesity on lung volumes. Thorax. 2014;69:752–9. doi: 10.1136/thoraxjnl-2014-205148. [DOI] [PubMed] [Google Scholar]
- 7.Ehrlich SF, Quesenberry CP, Jr, Van Den Eeden SK, Shan J, Ferrara A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care. 2010;33:55–60. doi: 10.2337/dc09-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vu T, Jin L, Datta PK. Effect of cigarette smoking on epithelial to mesenchymal transition (EMT) in lung cancer. J Clin Med. 2016;5 doi: 10.3390/jcm5040044. pii: E44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer BM, Pavlisko E, Voynow JA. Pathogenic triad in COPD: Oxidative stress, protease-antiprotease imbalance, and inflammation. Int J Chron Obstruct Pulmon Dis. 2011;6:413–21. doi: 10.2147/COPD.S10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milla CE, Zirbes J. Pulmonary complications of endocrine and metabolic disorders. Paediatr Respir Rev. 2012;13:23–8. doi: 10.1016/j.prrv.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Mir SH, Darzi MM. Histopathological abnormalities of prolonged alloxan-induced diabetes mellitus in rabbits. Int J Exp Pathol. 2009;90:66–73. doi: 10.1111/j.1365-2613.2008.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godo S, Shimokawa H. Divergent roles of endothelial nitric oxide synthases system in maintaining cardiovascular homeostasis. Free Radic Biol Med. 2016 doi: 10.1016/j.freeradbiomed.2016.12.019. pii: S0891-584931106-6. [DOI] [PubMed] [Google Scholar]
- 13.Arslan M, Akkurt I, Egilmez H, Atalar M, Salk I. Biomass exposure and the high resolution computed tomographic and spirometric findings. Eur J Radiol. 2004;52:192–9. doi: 10.1016/j.ejrad.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Moreira MA, Barbosa MA, Queiroz MC, Teixeira KI, Torres PP, Santana Júnior PJ, et al. Pulmonary changes on HRCT scans in nonsmoking females with COPD due to wood smoke exposure. J Bras Pneumol. 2013;39:155–63. doi: 10.1590/S1806-37132013000200006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casey G. COPD: Obstructed lungs. Nurs N Z. 2016;22:20–4. [PubMed] [Google Scholar]
- 16.Yeh HC, Punjabi NM, Wang NY, Pankow JS, Duncan BB, Cox CE, et al. Cross-sectional and prospective study of lung function in adults with type 2 diabetes: The Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. 2008;31:741–6. doi: 10.2337/dc07-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klekotka RB, Mizgala E, Król W. The etiology of lower respiratory tract infections in people with diabetes. Pneumonol Alergol Pol. 2015;83:401–8. doi: 10.5603/PiAP.2015.0065. [DOI] [PubMed] [Google Scholar]
- 18.Kaparianos A, Argyropoulou E, Sampsonas F, Karkoulias K, Tsiamita M, Spiropoulos K. Pulmonary complications in diabetes mellitus. Chron Respir Dis. 2008;5:101–8. doi: 10.1177/1479972307086313. [DOI] [PubMed] [Google Scholar]
- 19.Weynand B, Jonckheere A, Frans A, Rahier J. Diabetes mellitus induces a thickening of the pulmonary basal lamina. Respiration. 1999;66:14–9. doi: 10.1159/000029331. [DOI] [PubMed] [Google Scholar]
- 20.Attili AK, Kazerooni EA, Gross BH, Flaherty KR, Myers JL, Martinez FJ. Smoking-related interstitial lung disease: Radiologic-clinical-pathologic correlation. Radiographics. 2008;28:1383–96. doi: 10.1148/rg.285075223. [DOI] [PubMed] [Google Scholar]
- 21.Scullion JE, Vincent E. Erectile dysfunction in COPD: A hidden co-morbidity. Chron Respir Dis. 2016;13:3–4. doi: 10.1177/1479972315616932. [DOI] [PMC free article] [PubMed] [Google Scholar]


