Abstract
Introduction:
The renal nephrometry scoring (RNS) system enables prediction the feasibility of nephron-sparing surgery (NSS) in renal masses. There is insufficient data regarding the outcome of robot-assisted NSS in tumors with RNS ≥10. We reviewed the trifecta outcomes of patients undergoing robotic NSS with high RNS and compare it with tumors of low and intermediate RNS.
Materials and Methods:
Our prospectively maintained data of all robot-assisted NSS were reviewed, and those with RNS of ≥10 were identified. Patient data, outcomes and postoperative estimated glomerular filtration rate were compared between high, intermediate and low RNS patients.
Results:
In high RNS group, the mean age of the patients was 53 years (male:female = 15:3). Mean diameter of tumors was 6.28 cm (3.0–10.5 cm). Mean operative time was 173.61 ± 52.66 min and mean warm ischemia time was 27.85 ± 5.27 min. Mean estimated blood loss (EBL) was 363.89 ± 296.45 ml. Mean hospital length of stay was 5.39 ± 1.91 days (3–9 days). When compared with low and intermediate RNS, only EBL and need for pelvicalyceal system repair was significantly higher in high RNS group. Postoperative complications, renal function preservation and oncological outcomes at 3 months were comparable in all the three groups.
Conclusion:
Robot-assisted NSS is feasible with comparable outcomes in tumors with high RNS.
INTRODUCTION
Robotic nephron-sparing surgery (NSS) is becoming the preferred treatment modality for small renal masses (SRMs) with low and intermediate renal nephrometry score (RNS <7).[1,2] Tumors with high nephrometry score ≥10 more often undergo radical nephrectomy than NSS.[3] Recent advances in technical skills and rapid suturing provided by the robot has given the surgeons more confidence in operating such tumors.[4] There is a need for functional preservation to prevent long-term deterioration especially in elderly patients or patients with progressive renal disease[5] Although data on RNS >7 have been published widely, data regarding outcome of robot-assisted NSS in renal tumors with high RNS ≥10 is still limited.[6,7] With the advent of robotic surgery, there has been a paradigm shift from radical extirpation to NSS for renal tumors with high RNS while maintaining the trifecta outcomes.[8] We present our short-term experience of managing cases of high RNS using robotic assistance and compare it with low and intermediate RNS score patients of our cohort.
MATERIALS AND METHODS
Following the introduction of a da Vinci Si system at our institution in 2014, a prospective database was maintained for all robot-assisted NSS. In an Institutional Ethics Committee approved protocol, this database was reviewed. All patients had undergone a triphasic contrast-enhanced computerized tomography (CT) scan before surgery for assessment of tumor anatomy and vascular anomalies [Figure 1a–d]. A CT angiogram was obtained for larger and more vascular tumors to delineate the hilar vascular anatomy in detail. For each patient, the following variables were extracted from the database: age, gender, clinical tumor size, RNS, operative time (OT), warm ischemia time (WIT), pelvicalyceal system (PCS) repair, estimated blood loss (EBL), baseline and postoperative (1 week) serum creatinine and hemoglobin level, intraoperative and early postoperative complications. Serum creatinine and estimated glomerular filtration rate (eGFR) by Cockcroft–Gault formula were used for renal function estimation. The following pathological data were also retrieved: tumor size, and stage according to 2009 version of tumor, node and metastasis classification,[9] histological subtypes according to the World Health Organization classification,[10] nuclear grade according to the Fuhrman classification, and surgical margin status.
Figure 1.
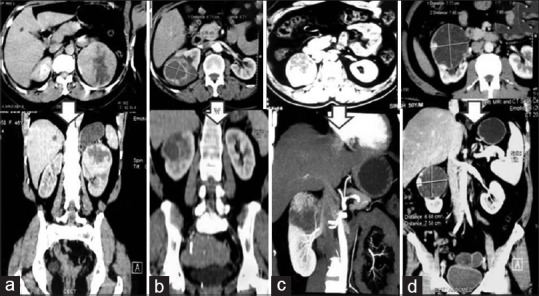
Representative computerized tomography images showing tumors with high RNSs. From left to right: (a) RNS = 11; (b) RNS = 11; (c) RNS = 10 and (d) RNS = 10. Above arrow - transverse sections; below arrow - coronal section images. RNS = Renal nephrometry scores
Patients were stratified according to the nephrometry scores and patients with RNS ≥10 were included for analysis. RNS was calculated by two urologists independently and in consensus whenever there was a difference of opinion. The patients were followed up at the end of 2 weeks with clinical examination. Complications were determined as per the Clavien-Dindo classification within a 30-day period. Clinical examination with hemogram and renal function test were done at 3 months follow-up. The eGFR was calculated and the change in eGFR was determined at 3 months. Follow-up imaging using CT scan was done at 6 months of follow-up.
Operative technique
The patient was positioned in the modified flank position at approximately 60°. Veress needle was used to create pneumoperitoneum. Port placement for right- and left-sided tumor is depicted in Figure 2. The robot was then docked from the patient's back, with the camera oriented in line with the kidney. The fourth robotic arm was used in only one case of purely endophytic tumor.
Figure 2.
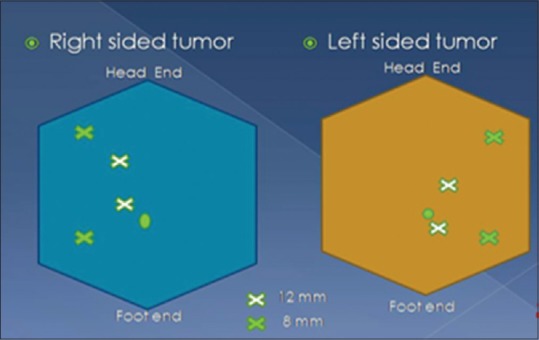
Port placement
A 0° scope was used, along with the 8 mm EndoWrist (Intuitive Surgical, Sunnyvale, CA, USA) monopolar scissors in the right arm and the 8 mm EndoWrist Maryland or ProGrasp grasper in the left arm. After mobilizing the colon, the hilum was dissected to delineate the renal vessels. Only arterial clamping was done routinely, and both artery and vein were clamped in one patient with a large bulky tumor.
Gerota's fascia was left atop the mass to assist in histopathologic staging and also to use for retraction. The laparoscopic ultrasound probe was used to plan the excision margins and the margins were scored to mark the resection boundaries.
The hilum was occluded by laparoscopic bulldog clamps and the tumor resected along the previously scored margin using monopolar scissors. PCS, if opened, was sutured using Vicryl 3-0 suture in a continuous fashion. PCS injury was identified by visual inspection only, and no special methodology was used for its identification. Renorrhaphy was done using the barbed 2-0 V-Loc Suture (Covidien, USA). These sutures were placed in a running horizontal mattress fashion and secured in place with Hem-o-lok clips in a sliding technique.
The specimen was placed in a custom made retrieval bag and removed from an extended lower quadrant port site. A drain was placed through a lower lateral port. The port site was closed using Vicryl 1-0 or port closure suture.
Statistical analysis
Continuous variables were reported as mean, median, and range. For all statistical analyses, a two-sided P < 0.05 was considered statistically significant. Data of all the patients were analyzed using Microsoft Excel and SPSS version 20.0 (IBM Corporation, New York, USA). Discrete categorical data were represented in the form of either a number or a percentage. Continuous data, assumed to be normally distributed, were written as in the form of its mean and standard deviation when it was skewed it was written in the form of its median and interquartile range, as per the requirement. The normality of quantitative data was checked by measures of Kolmogorov–Smirnov tests of normality. For Normally distributed data means of three groups of RNS (low, intermediate, and high) were compared using one-way ANOVA followed by post hoc multiple comparisons test. For skewed data Kruskal–Wallis test followed by Mann–Whitney test for two groups was applied.
RESULTS
Eighteen out of 79 cases met the criteria of high RNS. The mean age of the patients was 53 years (male:female = 15:3) and mean body mass index (BMI) was 25.62 ± 2.78. Mean diameter of the tumors was 6.28 cm (3.0–10.5 cm). Mean tumor size in high, intermediate, and low RNS group were 6.28 ± 2.18 cm, 4.96 ± 1.48 cm, and 3.73 ± 0.99 cm, respectively. Five patients had hypertension alone and one patient had diabetes alone while two patients had both. RNS was 12 in 1 patient, 11 in 5 patients, and 10 in remaining 12 patients. All 18 patients underwent NSS with robotic assistance with no conversion. Mean operating time (OT) was 173.61 ± 52.66 min (90–280 min). Mean WIT was 27.85 ± 5.27 min (18–40 min). Mean EBL was 363.89 ± 296.45 ml (50–1200 ml). Mean pre- and post-operative hemoglobin levels were 13.6 g/dl and 11.8 g/dl, respectively. PCS was opened in 15/18 (83.3%) cases. Double J stent was placed in two cases. Mean pre- and post-operative creatinine levels (1 week postoperatively) were 0.94 mg/dl (0.6–1.32) and 1.05 mg/dl (0.6–1.7), respectively. Mean hospital length of stay (LOS) was 5.39 ± 1.91 days (3–9 days). Eleven out of 18 (61.1%) tumors were clear cell carcinoma. Fuhrman grade was 1, 2, and 3 in four, six, and one patient, respectively. Margin status was positive in one case. Two patients each of oncocytoma, multicystic nephroma and one each of chromophobe, mucinous tubular and papillary variant of renal cell carcinoma were reported.
High RNS group data were compared with low and intermediate RNS groups [Table 1] BMI was significantly higher in high RNS group, than the low and intermediate groups. The mean OT (P = 0.667) and WIT (P = 0.211) were not significantly different among the three groups (low, intermediate, and high RNS). However, the EBL was significantly higher in high RNS group (P = 0.028). High RNS group had a significantly higher occurrence of pelvicalyceal entry than that in low and intermediate groups (P < 0.001). There was no significant difference in mean pre- and post-operative creatinine levels (P = 0.087). The baseline eGFR by Cockcroft–Gault formula was comparable in the three groups. The eGFR3/eGFR1 that is the ratio of latest eGFR (3 months postoperative) to baseline eGFR was also comparable among the three groups. Complications ranged from Clavein-Dindo Grade 1, 2 and 3 which were not significantly different among the groups.
Table 1.
Distribution as per RENAL scores of various parameters
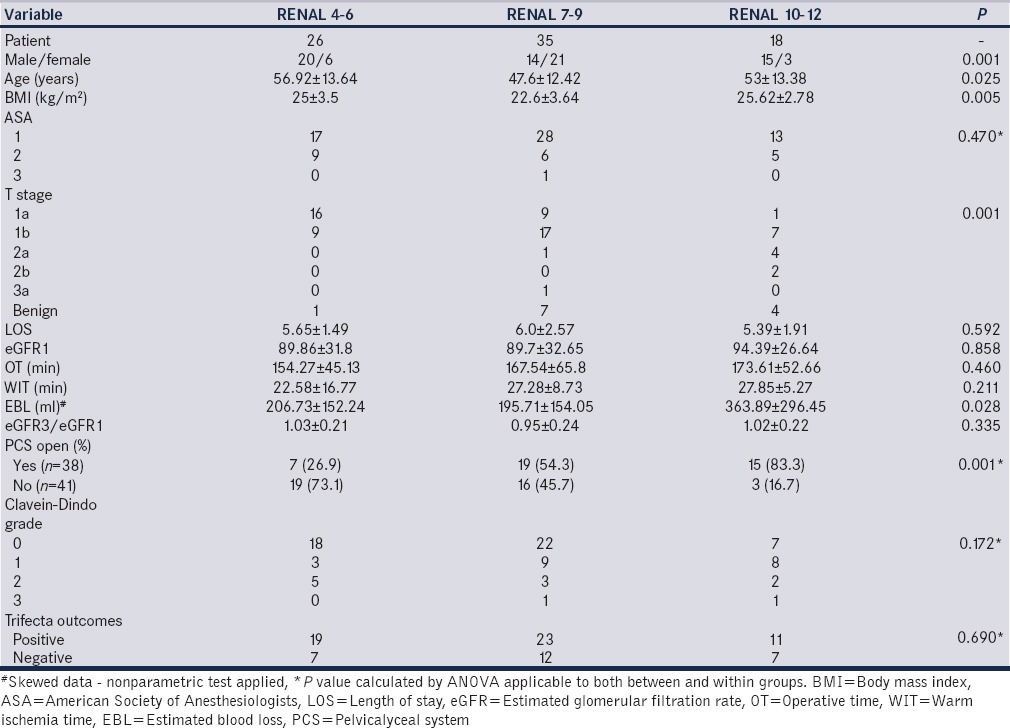
Overall, there was one recurrence at median follow-up of 8.71 ± 4.15 months (2–22 months) in high RNS group. Although a short-term comparison, the trifecta outcomes (WIT <30 min, Clavein-Dindo ≤ Grade 2 and negative surgical margins) were achieved in 61.1%, 65.7% and 73.1% of high, intermediate, and low RNS groups, respectively (P = 0.690).
DISCUSSION
Robotic NSS is becoming the preferred treatment modality for SRMs.[1,2] Robotic approach for partial nephrectomy has been found to be comparable to conventional laparoscopic approach in current literature.[8,11] With recent advances in technical skills and easier tumor handling followed by rapid suturing provided by robot, it has given the surgeons more confidence in operating upon tumors with high RNS.[4,12,13] Utilization rates of partial nephrectomy have been reported to be increasing for intermediate to high complex tumors.[6,12,13] Data regarding the outcome of robot-assisted NSS in renal tumors with high RNS ≥10 is still in infancy.[6,7,8] In the present study, patients with high RNS had higher blood loss and PCS violation as compared to the low and intermediate RNS group. However, the complications, hospital LOS, WIT, and OT and change in eGFR are not significantly different among the three groups. It also shows the feasibility of robotic NSS in achieving short-term reasonable trifecta outcomes in higher nephrometry score tumors.
Our technique was similar to that followed by the majority of the surgeons worldwide. We have found a few key points useful to decrease the OT and WIT. First, ensuring that in cases with high RNS, the presence of two expert console surgeons, one of whom became the patient side scrubbed surgeon during the clamp time. Another important point is that we clamped the renal artery only to save time, however, both arterial and venous clamping was used in a patient with very large tumor with proximity to the renal vein. The use of intraoperative ultrasound in all such cases to get adequate tumor-free margin. Finally, continuous suturing of parenchyma with V-loc barbed suture helped to achieve renorrhaphy at a faster pace as compared to conventional vicryl suture.
White et al. published data of 67 patients having RNS >7.[6] Out of 67 patients, 11 patients had high RNS ≥10. They found that the perioperative outcomes among the three groups showed a statistically significant difference in EBL, OT, and WIT in favor of the lower and moderately complex groups over the highly complex group. In a study by Rogers et al. who reported a multi-institutional analysis of robot-assisted NSS for renal hilar tumors (11 hilar tumors, RNS scores not defined).[14] Our perioperative results are also comparable to those of White et al.[6] and Rogers et al. [Table 2].[14] Another study was published by Masson-Lacomte et al. on robot-assisted NSS for tumors > 4 cm.[7] Only 2 patients were of RNS ≥ 10. They found that tumor size does not sufficiently discriminate complexity and RNS should be used to describe tumor complexity.
Table 2.
Comparison of perioperative measures following nephron sparing surgery for high complexity tumors
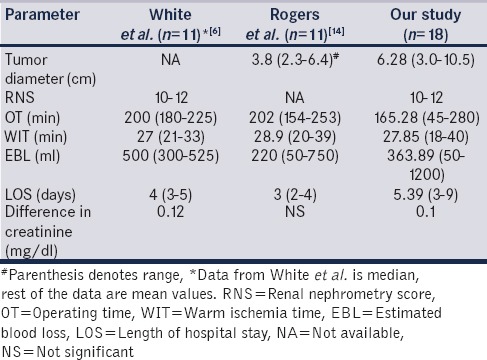
Trifecta outcome as defined by negative margin status, minimal complications and functional preservation remain in acceptable range. There has been one recurrence so far with the earliest patient completing 22 months and follow-up imaging showing no tumor. The WIT remains a consideration when operating on such complex tumors with data showing higher WIT while compared to low and intermediate complexity tumors.[6] However, the impact of WIT on long-term functional outcome still remains controversial, and the upper limit of 30 min is under the scanner with recent study extending the same up to 40 min.[15] Moreover, the WIT was well within the limit of 30 min for most of our cases.
Regarding the trifecta outcomes, Raheem et al. found that there was significant higher tumor size, OT, EBL, and Padua scores in trifecta negative patients.[16] They hypothesized that patient characteristics such as body habitus surgical history and difficulty of hilar dissection may be more influential determinants than tumor size and location. In this study, although the trifecta outcomes were achieved in 61% of high RNS patients it was not significantly different than the other two groups.
A recent study by Kopp et al. assessed renal functional outcomes after radical or partial nephrectomy for renal masses ≥7 cm. RNS ≥10 (odds ratio, 6.67; P = 0.025) and radical nephrectomy among patients with RNS <10 (odds ratio, 24.8; P < 0.001) were independently associated with de novo chronic kidney disease at 6 months by logistic regression analysis.[17] They concluded that radical nephrectomy is independently associated with decreased renal function compared to partial for T2 renal masses with RNS ≤10, but not >10. They further insisted that large multi-institutional and long-term data will be needed to prove the impact of parenchymal preservation on long-term functional preservation. Thus, these studies highlight that long-term benefit of performing NSS in RNS >10 tumors remains controversial and only long-term data would clarify this. Our study, however, shows the feasibility, and successful short-term outcomes, long-term follow-up of our study cohort and similar such studies would further clarify the utility of NSS in RNS >10.
The limitations of our study remain the small number of patients and short follow-up. However, the purpose of this study was to assess feasibility and reproducibility of performing such complex surgery, and we found that the short-term perioperative trifecta outcomes remain within acceptable limits. In our initial experience with the da Vinci Si, we found it useful in high RNS tumors as the perioperative outcomes and complications remain acceptable and comparable to the other two groups. The assessment of differential function will be of utmost importance in these patients so as to assess the compensatory mechanisms, effect of hyperfiltration injury and glomerulosclerosis in the remaining renal moiety. A similar assessment is planned for these patients. The proof whether saving the renal parenchyma is actually helpful in such complex tumors in true sense will be determined by the long-term studies with larger number of subjects and studies commenting on the true function of the remaining moiety. However, till these data are available, it is imperative to save as much renal parenchyma as possible in such patients.
CONCLUSION
Our data show that high RNS group has higher blood loss and PCS violation than the low and intermediate RNS group. However, the complications, LOS, WIT, and OT and change in eGFR are not significantly different among the three groups. It also shows the feasibility of robotic NSS in achieving short-term reasonable trifecta outcomes in higher nephrometry score tumors. This study reinforces the reproducibility of robot-assisted NSS for complex renal tumors. Long-term data with functional studies on larger number of patients will be needed to prove the long-term benefit of this conscientious exercise.
Acknowledgment
We acknowledge Dr. Muralidaran C. from Department of Pathology, PGIMER, Chandigarh for reviewing the histopathology slides of all the cases.
Footnotes
Financial support and sponsorship: Nil.
Conflicts of interest: There are no conflicts of interest.
REFERENCES
- 1.Scoll BJ, Uzzo RG, Chen DY, Boorjian SA, Kutikov A, Manley BJ, et al. Robot-assisted partial nephrectomy: A large single-institutional experience. Urology. 2010;75:1328–34. doi: 10.1016/j.urology.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benway BM, Bhayani SB, Rogers CG, Porter JR, Buffi NM, Figenshau RS, et al. Robot-assisted partial nephrectomy: An international experience. Eur Urol. 2010;57:815–20. doi: 10.1016/j.eururo.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: A comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182:844–53. doi: 10.1016/j.juro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 4.Mottrie A, De Naeyer G, Schatteman P, Carpentier P, Sangalli M, Ficarra V. Impact of the learning curve on perioperative outcomes in patients who underwent robotic partial nephrectomy for parenchymal renal tumours. Eur Urol. 2010;58:127–32. doi: 10.1016/j.eururo.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 5.Chiu Y, Chiu AW. Renal preservation therapy for renal cell carcinoma. Int J Surg Oncol 2012. 2012:123596. doi: 10.1155/2012/123596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White MA, Haber GP, Autorino R, Khanna R, Hernandez AV, Forest S, et al. Outcomes of robotic partial nephrectomy for renal masses with nephrometry score of ≥7. Urology. 2011;77:809–13. doi: 10.1016/j.urology.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Masson-Lecomte A, Yates DR, Bensalah K, Vaessen C, de la Taille A, Roumiguié M, et al. Robot-assisted laparoscopic nephron sparing surgery for tumors over 4 cm: Operative results and preliminary oncologic outcomes from a multicentre French study. Eur J Surg Oncol. 2013;39:799–803. doi: 10.1016/j.ejso.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Masson-Lecomte A, Bensalah K, Seringe E, Vaessen C, de la Taille A, Doumerc N, et al. A prospective comparison of surgical and pathological outcomes obtained after robot-assisted or pure laparoscopic partial nephrectomy in moderate to complex renal tumours: Results from a French multicentre collaborative study. BJU Int. 2013;111:256–63. doi: 10.1111/j.1464-410X.2012.11528.x. [DOI] [PubMed] [Google Scholar]
- 9.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, et al. AJCC Cancer Staging Manual. 7th ed. New York: Springer-Verlag; 2010. [Google Scholar]
- 10.Eble JN, Sauter G, Epstein JI, Sesterhenn IA, editors. World Health Organization Classification of Tumours, Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon: IARC Press; 2004. [Google Scholar]
- 11.Benway BM, Bhayani SB, Rogers CG, Dulabon LM, Patel MN, Lipkin M, et al. Robot assisted partial nephrectomy versus laparoscopic partial nephrectomy for renal tumors: A multi-institutional analysis of perioperative outcomes. J Urol. 2009;182:866–72. doi: 10.1016/j.juro.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 12.Borghesi M, Schiavina R, Gan M, Novara G, Mottrie A, Ficarra V. Expanding utilization of robotic partial nephrectomy for clinical T1b and complex T1a renal masses. World J Urol. 2013;31:499–504. doi: 10.1007/s00345-013-1095-2. [DOI] [PubMed] [Google Scholar]
- 13.Lane BR, Golan S, Eggener S, Tobert CM, Kahnoski RJ, Kutikov A, et al. Differential use of partial nephrectomy for intermediate and high complexity tumors may explain variability in reported utilization rates. J Urol. 2013;189:2047–53. doi: 10.1016/j.juro.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Rogers CG, Metwalli A, Blatt AM, Bratslavsky G, Menon M, Linehan WM, et al. Robotic partial nephrectomy for renal hilar tumors: A multi-institutional analysis. J Urol. 2008;180:2353–6. doi: 10.1016/j.juro.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godoy G, Ramanathan V, Kanofsky JA, O’Malley RL, Tareen BU, Taneja SS, et al. Effect of warm ischemia time during laparoscopic partial nephrectomy on early postoperative glomerular filtration rate. J Urol. 2009;181:2438–43. doi: 10.1016/j.juro.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Abdel Raheem A, Alatawi A, Kim DK, Sheikh A, Alabdulaali I, Han WK, et al. Outcomes of high-complexity renal tumours with a Preoperative Aspects and Dimensions Used for an Anatomical (PADUA) score of ≥10 after robot-assisted partial nephrectomy with a median 46.5-month follow-up: A tertiary centre experience. BJU Int. 2016;118:770–8. doi: 10.1111/bju.13501. [DOI] [PubMed] [Google Scholar]
- 17.Kopp RP, Liss MA, Mehrazin R, Wang S, Lee HJ, Jabaji R, et al. Analysis of renal functional outcomes after radical or partial nephrectomy for renal masses ≥7 cm using the RENAL score. Urology. 2015;86:312–9. doi: 10.1016/j.urology.2015.02.067. [DOI] [PubMed] [Google Scholar]


