Highlights
-
•
Colorectal metastases to thyroid are rare.
-
•
The majority of the cases of colorectal thyroid metastases are diagnosed lately in the evolution of a known malignancy.
-
•
A low threshold of suspicion is crucial to make a timely diagnosis in order to avoid its high morbidity.
-
•
Colorectal thyroid metastization could be clinically silent and a raise in tumoral markers could be the only sign.
-
•
Treatment is controversial and the prognosis is usually poor. Without surgery, the need may arise for tracheostomy.
Keywords: Colorectal cancer, Thyroid, Metastasis, Case report
Abstract
Introduction
Thyroid metastases from colorectal cancer are uncommon and few cases are described in literature.
Case presentation
A 64-year-old female patient presented with an asymptomatic right cervical nodule with a rapid growth six years after sigmoidectomy for cancer and two years after resection of colorectal lung metastases. Increased CA 19.9 was identified and a thoracoabdominal CT scan revealed the onset of new metastatic bilateral pulmonary lesions. Neck ultrasonography showed a suspicious nodule in the right thyroid lobe, and Fine-needle Aspiration Cytology (FNAC) of the nodule lead to the diagnosis of colorectal cancer metastasis. A right thyroid lobectomy with right central lymph node dissection was performed. The patient underwent chemotherapy with response, but this was posteriorly suspended due to haematological side effects, and the disease spread.
Discussion
Thyroid metastases from colorectal cancer are rare, but, with the improvement of radiologic exams and the higher survival rate of these patients, more cases are being described. The majority of the cases present pulmonary and hepatic metastases and the prognosis is poor. The decision to operate and the type of operation depend on the extent of the metastatic disease and the patient’s overall condition.
Conclusion
A low threshold of suspicion is crucial to make a timely diagnosis of thyroid metastases from colorectal cancer. Treatment is controversial, but, without surgery, the need may arise for tracheostomy.
1. Introduction
Colorectal cancer is the second most common cancer worldwide and the second deadliest in Europe. If metastases are present, prognosis is poor [1]. Approximately 20% of patients with colon cancer have metastases at diagnosis, and the most common sites are: liver, lungs and peritoneum [2].
Thyroid metastases are rare. In autopsy series, thyroid metastases are mainly from lung cancer, whereas in clinical series, renal cell carcinoma is the most frequent cause [3]. Thyroid metastases from colorectal cancer are even rarer and occur late in the disease course. Liévre et al. identified 6 cases among 5862 patients with colorectal cancer (0.1%) between 1993 and 2004 [4].
We report a case of a patient with colon cancer with lung metastases who was diagnosed with a thyroid metastasis six years after the first cancer treatment.
This study has been reported in compliance with the SCARE criteria [5].
2. Case presentation
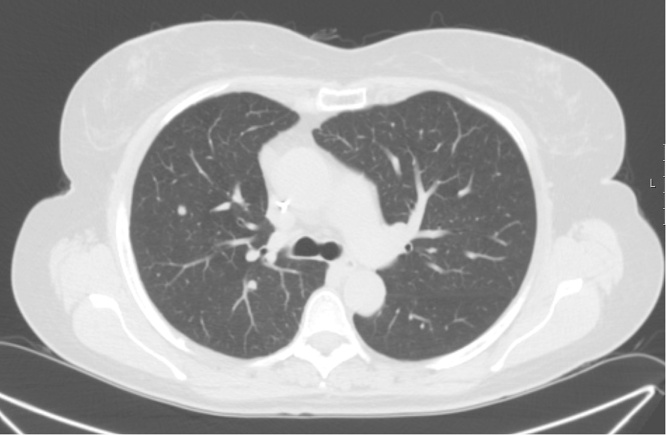
A 64-year-old female patient was submitted to sigmoidectomy in 2009 for sigmoid cancer, followed by adjuvant chemotherapy. The postoperative pathological diagnosis was a moderately differentiated sigmoid adenocarcinoma with regional ganglion metastases pT3 N1 M0, stage IIIB, KRAS wild type. In 2011, a right pulmonary metastasis was diagnosed and pulmonary metastasectomy was conducted. Two years later, a new lung metastasis was found on follow-up CT scan and a right inferior pulmonary lobectomy was performed, followed by adjuvant chemotherapy with FOLFOX. In 2015, an asymptomatic right cervical bump with rapid growth, without apparent cervical adenopathies, was detected. Additionally, an increasing CA 19.9 (57 ng/mL (<37)) with a normal CEA (5.14 ng/mL (<5.4)) was determined. Blood tests revealed no anaemia, but normal liver, renal and thyroid functions. A Thoracoabdominal CT scan was performed, revealing the onset of new metastatic pulmonary lesions in both lungs. No abdominal alterations were observed. In addition to confirming these pulmonary lesions, a PET scan diagnosed an enlargement of the thyroid gland due to a hypodense and hypermetabolic nodule in the right thyroid lobe measuring 38 × 21 × 45 mm. Cervical ultrasonography (US) showed a heterogeneous hypoechoic nodule with calcifications measuring 33 × 27 × 25 mm in the right thyroid lobe (Fig. 1) and a right internal jugular adenopathy (11 × 6 mm). FNAC of the nodule diagnosed a metastasis from colorectal cancer. At this time, CEA was increasing (9.5 ng/dL). Right thyroid lobectomy with right central lymph node dissection was performed (Fig. 2, Fig. 3). Surgical resection was difficult due to the strong adhesions of the tumor to the adjacent structures, namely the cricoid cartilage and cricopharyngeus muscle. No surgical complications occurred and the patient was discharged home 3 days after surgery. The pathology report revealed a tangential excision with the surgical margin of a diffuse carcinoma invasion with uncompleted glandular cell elements and associated necrosis infiltrating the thyroid capsule and surrounding muscular and fibroadipose tissues, as well as three metastatic adenopathies. Immunohistochemistry was positive for gastrointestinal markers CDX2 and CK20 and negative for CK7 and TTF-1, thus confirming colorectal origin. Adjuvant chemotherapy with FOLFIRI and Cetuximab was initiated. However, therapy was interrupted after six cycles due to haematological side effects and severe asthenia. At this time, the metastatic disease was limited to one right pulmonary nodule, with CEA and CA- 19.9 within normal values. Ten months after surgery and four months after chemotherapy suspension, hoarseness and intermittent dysphagia with recurrence of right cervical mass occurred. CEA and CA 19.9 levels were increased. The CT and PET scan revealed extensive right cervical metastatic disease with compression of the right internal jugular vein (Fig. 4) and bilateral pulmonary metastases (Fig. 5). Palliative cervical radiotherapy was not successful and the disease spread to the pleura, pericardium, liver and peritoneum. Palliative chemotherapy with capecitabine was initiated and fifteen months after thyroid surgery patient is still alive.
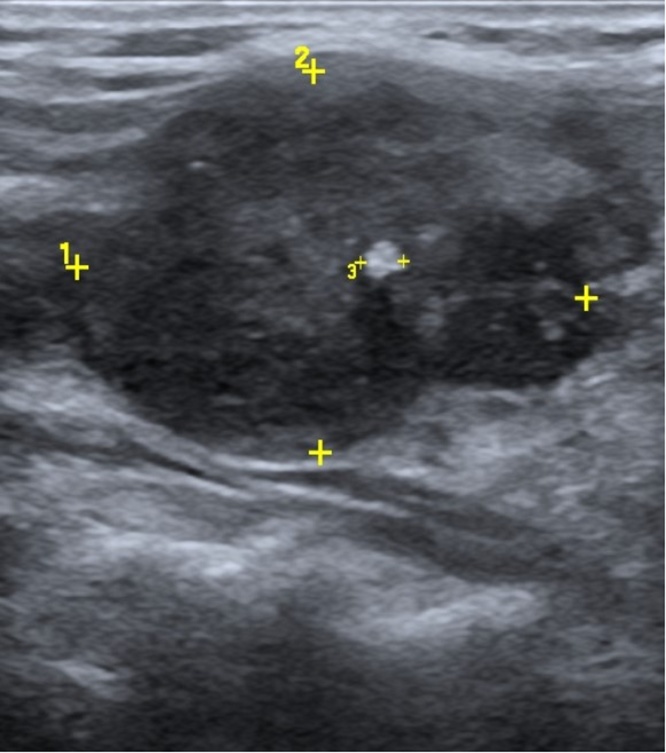
Fig. 1.
Cervical Ultrasonography: a heterogeneous hypoechoic nodule with calcifications measuring 33 × 27 × 25 mm in the right thyroid lobe.
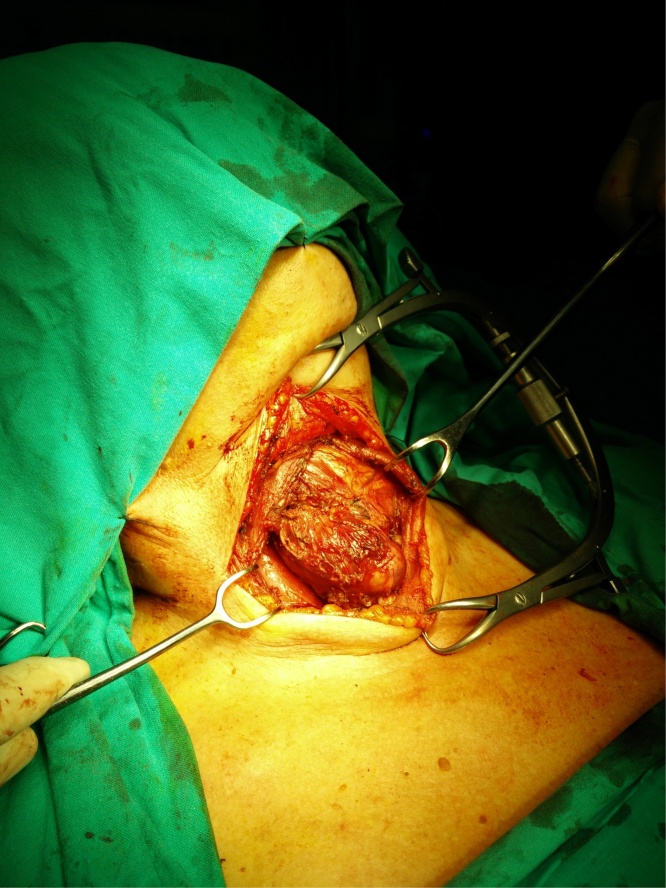
Fig. 2.
Intraoperative image from the right thyroid mass showing intense adhesions to the adjacent structures.
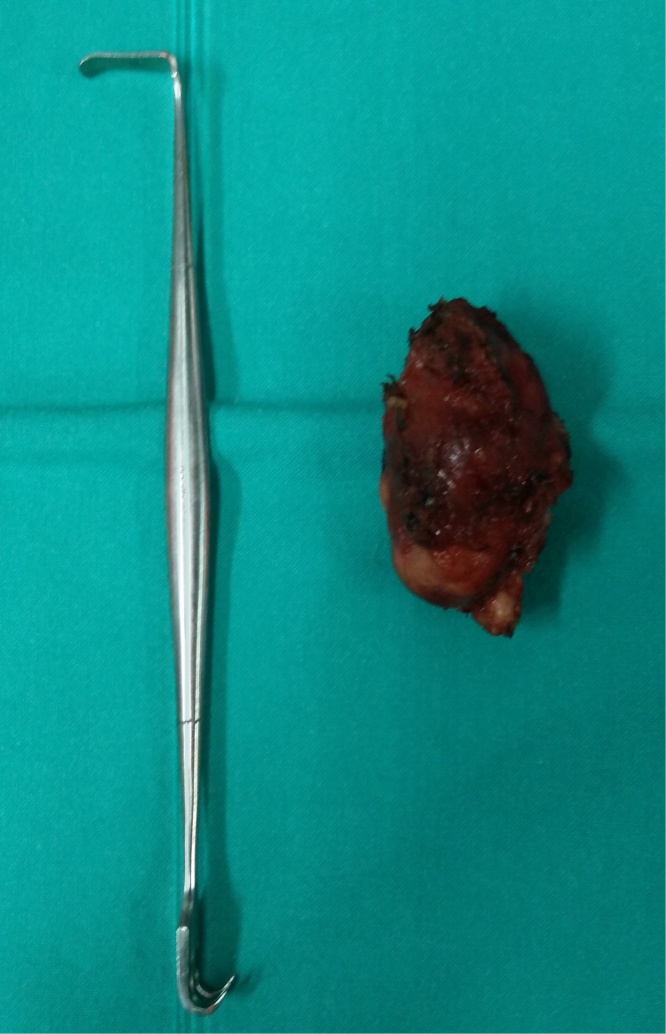
Fig. 3.
Macroscopic aspect of the right thyroid lobe after excision.
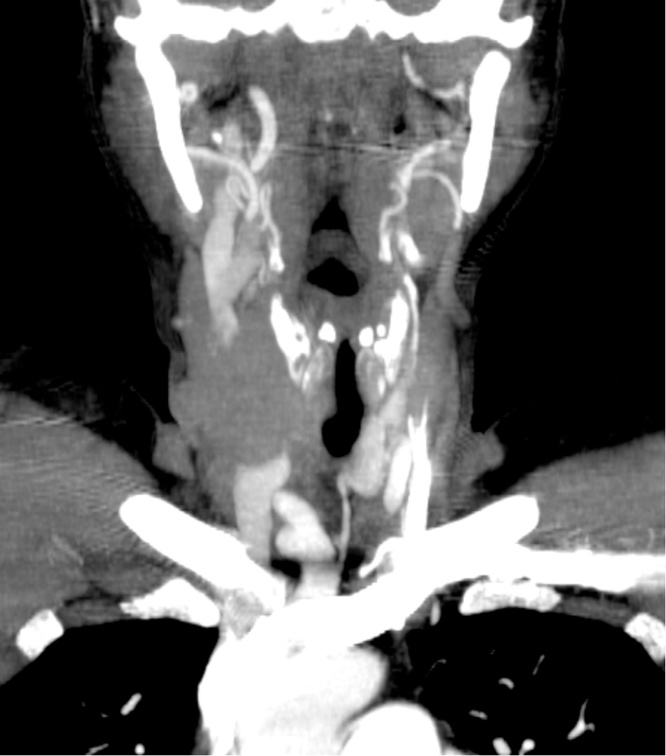
Fig. 4.
Cervical CT: extensive right cervical metastatic disease with compressing right jugular internal vein.
Fig. 5.
Thoracic CT: right metastatic pulmonary nodules.
3. Discussion
Metastases to the thyroid gland from non-thyroidal sites are an uncommon clinical presentation. In autopsy series, the lung is the most common site of primary tumor metastatic to the thyroid, whereas in clinical series, renal cell carcinoma is the most frequent, followed by breast and gastrointestinal neoplasms [6]. According to Nixon et al., the high oxygen and iodine environment may impair the ability of metastatic cells to settle and develop in the thyroid. Additionally, the fast blood flow could make adhesion and implantation of tumor cells difficult [7].
Although thyroid metastases from colorectal cancer are rare, more cases are being described due to the emergence of more accurate image exams and the higher survival rate of patients. Lievre et al. described 6 cases (0.1%) of thyroid metastases among 5862 patients with colorectal cancer between January 1993 and June 2004 [4]. In 2013, Froylich et al. reviewed metachronous colon metastases to the thyroid and found 34 cases. In this study, two thirds of the patients were female, which suggests hormonal influence; the primary sites were the rectum (41%), the sigmoid colon (33%), the right colon (19%) and the left colon (11%); 75% of the patients had stage III or IV colon cancer; metastases to the thyroid were diagnosed 6 months to 8 years after colonic resection; and, in 29 patients, there was involvement of another organ [8]. Even though metastases to the thyroid might be the initial presentation of an occult primary tumor (20–40%), the majority of the cases are diagnosed in the setting of a known malignancy (60–80%) [4], [6], [9], [10], [11]. Therefore, the possibility of a metastasis to the thyroid should be considered in patients presenting a solitary thyroid nodule and a previous history of cancer [7,12]. Moreover, there is usually evidence of previous or concomitant pulmonary and/or hepatic secondary disease, which favors a hematogenous metastatic route [9], [13]. However, there are reports of right colon carcinoma with thyroid metastases without liver disease, which could be explained by the drainage of tumor cells to the inferior vena cava through the vertebral vessels [8]. In the reported case, the patient was a female with a past history of stage III sigmoid carcinoma with pulmonary metastases who was diagnosed with a thyroid metastasis 6 years after colon resection.
Most patients with metastases to the thyroid are asymptomatic (31%) and the most common presentation is swelling or a cervical mass (26%) [14]. Lièvre et al. report that 50% of the patients were asymptomatic and the metastases were diagnosed by follow-up exams, and the remaining 50% had goiter-related symptoms [4]. Thyroid dysfunction is rare and usually appears late with thyrotoxicosis [6,15]. An increase in tumor markers (mostly CEA) is common and can be the first sign [16]. US, CT, magnetic resonance imaging (MRI) and PET are used to study thyroid nodules but only FNAC can differentiate a primary thyroid lesion from a metastasis, with positive and negative predictive values of 89 and 93%, respectively [6]. Nevertheless, not all cases of thyroid metastases are diagnosed, thus emphasizing the importance of a low threshold of suspicion [17]. According to some authors, increased thyroid 18-FDG uptake in the PET scan can be the first and only sign of thyroid neoplasm (including a colon metastasis). Hence, its use is advised in the initial staging of colorectal cancer [16], [17], [18]. Additionally, immunohistochemistry is crucial for the final diagnosis. Primary thyroid tumors are positive for CK7 and negative for CK20, in contrast to colon cancer metastases, which are positive for CK20 and negative for CK7 [6,11]. In line with literature data, our patient presented increased tumor markers without symptoms, and only four months later was the cervical nodule noticed by the patient. The diagnosis for colorectal cancer metastasis was then confirmed by cytology and immunohistochemistry (FNAC).
The decision to operate depends both on the extent of the metastatic disease in other sites and the medical condition of the patient [14], [17], [19]. Surgical treatment prevents asphyxiation [19], thus avoiding an emergent tracheostomy [20], [21], [22], [23]. However, the extent of surgical resection is not well defined, with some groups recommending total thyroidectomy based on the high rates of multicentric disease [6], [14], [19]. Although thyroid lobectomy alone could be associated with positive margins and therefore favor total thyroidectomy, this is not mandatory as long as adequate margins are achieved with lobectomy. Additionally, metastases to the thyroid gland are not sensitive to radioactive iodine, hence making total thyroidectomy not mandatory for this purpose [6]. Ten percent of the patients with metastases to the thyroid are found to be unresectable intra-operatively, and those who are resectable develop metastatic diseases in other sites [7]. Concomitant regional lymph node involvement is rare, therefore prophylactic neck dissection is not recommended [6]. However, Lièvre et al. advocate lymph node dissection, for they found lymph node metastases in all patients submitted to thyroidectomy with central and lateral neck lymph node dissection [4].
Chemotherapeutic drugs do not seem to have a good penetration into the thyroid gland, thus possibly making it a sanctuary for occult metastases [8]. A paper from Malani et al. supports these ideas [17], but there are other recent reports suggesting that combination chemotherapy is beneficial [22], and that radiotherapy may be advocated in case of life-threatening or disabling symptoms (e.g. dyspnea, dysphagia) [4]. In the presented case, we performed a right lobectomy because there was no evidence of contralateral disease, as well as a right central node dissection due to suspicion of lymph node metastasis.
Prognosis depends on the grade of malignancy of the primary lesion and whether there are metastases in other organs rather than the thyroid [13], [21]. While Montero et al. report a cancer-related death rate of 50% in less than a year [24], Lievre et al. report a median overall survival of 12 months (8–18 months) and a postoperative survival of 10 months (which is lower than after resection of lung or liver metastases), with two patients dying from metastatic recurrence in other sites, hence suggesting that total thyroidectomy should only be indicated in selected cases [4]. Conservative management seems to be the most logical approach in patients with multi-metastatic disease [4].
In the reported case, the disease seemed to be controlled after surgery and chemotherapy. However, due to side effects, chemotherapy was stopped and the disease spread rapidly. There was evidence of metastatic disease on cervical lymph nodes, lungs, pleura, mediastinum, liver and peritoneum one year after surgery.
4. Conclusion
The prevalence of thyroid metastases from colorectal cancer may be underestimated, as many patients are asymptomatic and these metastases are not commonly searched for. The possibility of metastases should be considered in patients presenting a solitary thyroid nodule and a past history of cancer. A low threshold of suspicion is crucial to make a timely diagnosis of thyroid metastases from colorectal cancer. Treatment is controversial, but, without surgery, the need may arise for tracheostomy.
Conflicts of interest
Authors declare no conflicts of interest
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Ethical approval was not needed since this paper describes the use of a well-known technique. Thyroid lobectomy is a valid option to treat thyroid colon metastases.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
M.I. Coelho – study concept and design, data collection and analysis, writing the paper, review.
M.N. Albano – data analysis, writing the paper, review.
C.E.C. Almeida – review.
N. Moreira – review.
Guarantor
Luís S. Reis; Carlos M. Costa Almeida
Contributor Information
M.I. Coelho, Email: coelho.m.ines@gmail.com.
M.N. Albano, Email: miguelalbano@gmail.com.
C.E. Costa Almeida, Email: carloscostaalmeida@yahoo.com.
L.S. Reis, Email: lfrs.reis@gmail.com.
N. Moreira, Email: nidia.moreira.22@gmail.com.
C.M.C. Almeida, Email: c.m.costa.almeida@gmail.com.
References
- 1.Ait Ouakrim D., Pizot C., Boniol M., Malvezzi M., Boniol M., Negri E., Bota M., Jenkins M.A., Bleiberg H., Autier P. Trends in colorectal cancer mortality in Europe: retrospective analysis of the WHO mortality database. BMJ. 2015:h4970. doi: 10.1136/bmj.h4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riihimäki M., Hemminki A., Sundquist J., Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 2016;6:29765. doi: 10.1038/srep29765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willis R.A. Metastatic tumours in the thyreoid gland. Am. J. Pathol. 1931;7(3):187–208. http://www.ncbi.nlm.nih.gov/pubmed/19969962 (Accessed March 4, 2017) [PMC free article] [PubMed] [Google Scholar]
- 4.Lièvre A., Leboulleux S., Boige V., Travagli J.P., Dromain C., Elias D., Ducreux M., Malka D. Thyroid metastases from colorectal cancer: The Institut Gustave Roussy experience. Eur. J. Cancer. 2006;42:1756–1759. doi: 10.1016/j.ejca.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 5.Agha R.A., Fowler A.J., Saeta A., Barai I., Rajmohan S., Orgill D.P., Afifi R., Al-Ahmadi R., Albrecht J., Alsawadi A., Aronson J., Ather M.H., Bashashati M., Basu S., Bradley P., Chalkoo M., Challacombe B., Cross T., Derbyshire L., Farooq N., Hoffman J., Kadioglu H., Kasivisvanathan V., Kirshtein B., Klappenbach R., Laskin D., Miguel D., Milburn J., Mousavi S.R., Muensterer O., Ngu J., Nixon I., Noureldin A., Perakath B., Raison N., Raveendran K., Sullivan T., Thoma A., Thorat M., Valmasoni M., Massarut S., D’cruz A., Baskaran V., Giordano S., Roy G., Machado- Aranda D., Healy D., Carroll B., Rosin D. The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Nixon I.J., Coca-Pelaz A., Kaleva A.I., Triantafyllou A., Angelos P., Owen R.P., Rinaldo A., Shaha A.R., Silver C.E., Ferlito A. Metastasis to the thyroid gland: a critical review. Ann. Surg. Oncol. 2016;21 doi: 10.1245/s10434-016-5683-4. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nixon I.J., Whitcher M., Glick J., Palmer F.L., Shaha A.R., Shah J.P., Patel S.G., Ganly I. Surgical management of metastases to the thyroid gland. Ann. Surg. Oncol. 2011;18:800–804. doi: 10.1245/s10434-010-1408-2. [DOI] [PubMed] [Google Scholar]
- 8.Froylich D., Shiloni E., Hazzan D. Metachronous colon metastasis to the thyroid: a case report and literature review. Case Rep. Surg. 2013;2013:1–5. doi: 10.1155/2013/241678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumamoto K., Utsumi Y., Sugano K., Hoshino M., Suzuki S., Takenoshita S. Colon carcinoma metastasis to the thyroid gland: report of a case with a review of the literature. Tumori. 2006;92:252–256. doi: 10.1177/030089160609200314. http://www.ncbi.nlm.nih.gov/pubmed/16869247 (Accessed February 7, 2017) [DOI] [PubMed] [Google Scholar]
- 10.Cavanna L., Anselmi E., Palladino M., Pagani R. Colon carcinoma metastasis to the thyroid gland. Tumori. 2006;92:467. doi: 10.1177/030089160609200522. http://www.ncbi.nlm.nih.gov/pubmed/17168448 (Accessed February 7, 2017) [DOI] [PubMed] [Google Scholar]
- 11.Minami S., Inoue K., Irie J., Mine T., Tada N., Hirabaru M., Noda K., Ito S., Haraguchi M. Metastasis of colon cancer to the thyroid and cervical lymph nodes: a case report. Surg. Case Rep. 2016;2(108) doi: 10.1186/s40792-016-0237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roloff G.W., Yang Z., Wood L.V., Neychev V.K. Colon cancer metastasis to the thyroid gland: report of a case with unique molecular profile Key Clinical Message. Clin. Case Rep. 2016;4:549–553. doi: 10.1002/ccr3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura K., Nozawa K., Aoyagi Y., Ishihara S., Matsuda K., Fukushima J., Watanabe T. A case report of thyroid gland metastasis associated with lung metastasis from colon cancer. Tumori. 2011;97:229–332. doi: 10.1177/030089161109700217. [DOI] [PubMed] [Google Scholar]
- 14.Romero Arenas M.A., Ryu H., Lee S., Morris L.F., Grubbs E.G., Lee J.E., Perrier N.D. The role of thyroidectomy in metastatic disease to the thyroid gland. Ann. Surg. Oncol. 2014;21:434–439. doi: 10.1245/s10434-013-3282-1. [DOI] [PubMed] [Google Scholar]
- 15.Youn J.C., Rhee Y., Park S.Y., Kim W.H., Kim S.J., Chung H.C., Hong S.W., Lim S.-K. Severe hypothyroidism induced by thyroid metastasis of colon adenocarcinoma: a case report and review of the literature. Endocr. J. 2006;53:339–343. doi: 10.1507/endocrj.k05-115. http://www.ncbi.nlm.nih.gov/pubmed/16714841 (Accessed February 9, 2017) [DOI] [PubMed] [Google Scholar]
- 16.Iguchi T., Matsuoka J., Sato S., Okumura Y., Omori M., Mifune H., Akaki S., Kanazawa S. F-18 FDG PET demonstration of a thyroid metastasis in a patient with colon cancer. Clin. Nucl. Med. 2007;32:361–362. doi: 10.1097/01.rlu.0000259625.74256.01. [DOI] [PubMed] [Google Scholar]
- 17.Malani A.K., Gupta C., Rangineni S., Gupta V. Thyroid metastasis from colorectal cancer: role of [18F]-fluoro- 2-deoxy- D-glucose positron emission tomography. Clin. Colorectal Cancer. 2005;5:287–291. doi: 10.3816/ccc.2005.n.042. http://www.ncbi.nlm.nih.gov/pubmed/16356308 (Accessed February 9, 2017) [DOI] [PubMed] [Google Scholar]
- 18.Hanna W.C., Ponsky T.A., Trachiotis G.D., Knoll S.M. Colon cancer metastatic to the lung and the thyroid gland. Arch. Surg. 2006;141:93–96. doi: 10.1001/archsurg.141.1.93. [DOI] [PubMed] [Google Scholar]
- 19.Nixon I.J., Whitcher M., Glick J., Palmer F.L., Shaha A.R., Shah J.P., Patel S.G., Ganly I. Surgical management of metastases to the thyroid gland. Ann. Surg. Oncol. 2011;18:800–804. doi: 10.1245/s10434-010-1408-2. [DOI] [PubMed] [Google Scholar]
- 20.Alherabi A.Z., Marglani O.A., Gazzaz M.J., Abbas M.M. Colon cancer metastasis to the thyroid gland. Saudi Med. J. 2014;35:868–871. http://www.ncbi.nlm.nih.gov/pubmed/25129189 (Accessed February 7, 2017) [PubMed] [Google Scholar]
- 21.Goatman C., Goldsmith P.J., Antonopoulos V., Ali B. Metastasis of colorectal adenocarcinoma to the thyroid: a case report and review of the literature. Case Rep. Surg. 2012;2012:1–3. doi: 10.1155/2012/179407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung W.Y., Brierley J., Mackay H.J. Treatment of rectal cancer metastases to the thyroid gland: report of two cases. Clin. Colorectal Cancer. 2008 Jul;7(4):280–282. doi: 10.3816/CCC.2008.n.036. [DOI] [PubMed] [Google Scholar]
- 23.Poon D., Toh H.C. Sim CS: Two case reports of metastases from colon carcinoma to the thyroid. Ann. Acad. Med. Singapore. 2004;33(January (1)):100–102. [PubMed] [Google Scholar]
- 24.Montero P.H., Ibrahimpasic T., Nixon I.J., Shaha A.R. Thyroid metastasectomy. J. Surg. Oncol. 2014:36–41. doi: 10.1002/jso.23452. [DOI] [PubMed] [Google Scholar]