Fig. 1. Homeostatic paracrine and endocrine signaling pathways in the adrenal cortex.
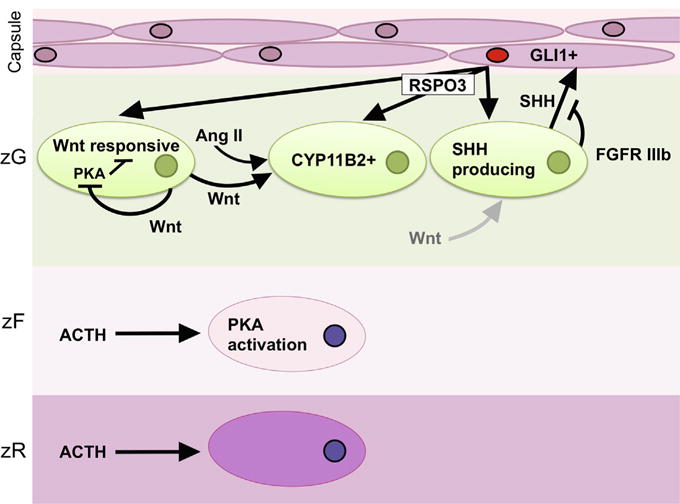
Sonic hedgehog (SHH) ligand is produced by clusters of cells in the zG (SHH producing cells), which serve as a stem/progenitor population for the embryonic and postnatal adrenal cortex. SHH acts on capsular cells, causing GLI1 expression and activation (GLI1+). Signaling downstream of FGFR IIIb in subcapsular zG cells is thought to reduce SHH signaling in the embryonic mouse. GLI1+ cells are also putative stem/progenitors that populate the adrenal cortex embryonically and post-natally in the mouse. Wnt responsive cells are those with active canonical Wnt signaling. WNT4 ligand, an effector and presumptive target of canonical Wnt signaling, acts on zG cells that secrete aldosterone in response to angiotensin II (Ang II) levels. WNT4 expression increases CYP11B2 expression and aldosterone levels. In the absence of WNT4 or in the presence of increased ACTH stimulation, PKA inhibits canonical Wnt signaling. RSPO3 ligand potentiates Wnt signaling, and is necessary for SHH, WNT4, and CYP11B2 expression both embryonically and in the post-natal adrenal. The majority of RSPO3 is produced by capsular GLI1+ cells. Known mechanisms of RSPO3 are mediated by the presence of Wnt ligands, though significant involvement of specific Wnts on SHH-producing cells (indicated by the grey arrow) has not been demonstrated in the adrenal cortex. In the zF, ACTH stimulates PKA activity, which is associated with growth and cortisol production in ACTs.
