Figure 4.
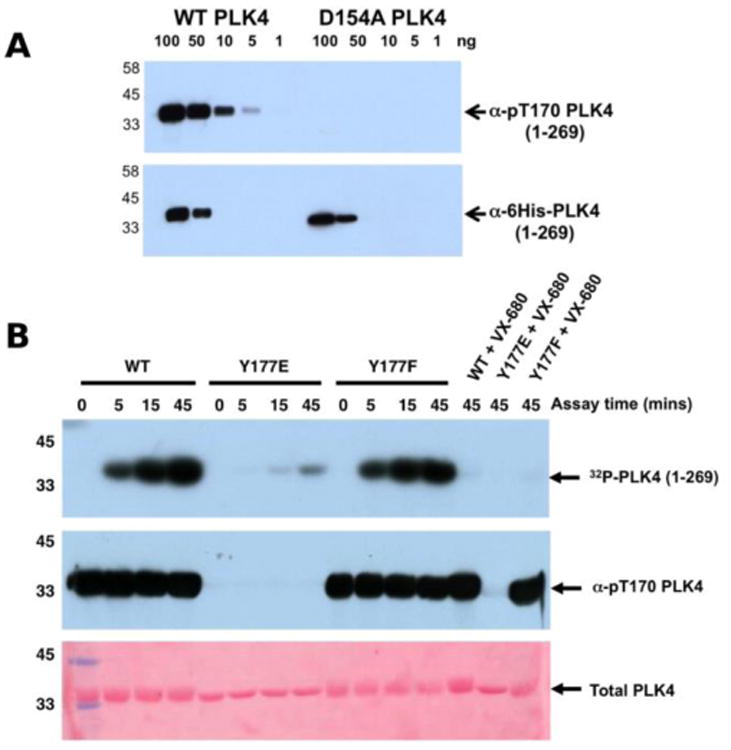
A) Wild type PLK4 (1-269) or D154APLK4 mutant PLK4 (1-296) were serially diluted and the indicated amounts (ng) were separated by SDS PAGE. Proteins were transferred to a nitrocellulose membrane and probed with rabbit pT170PLK4 PLK4 or 6His antibodies. Antibody binding was visualised using goat anti-rabbit secondary antibodies attached to HRP by ECL. B) PLK4 Y177EPLK4 mutation greatly reduces 32P incorporation (autophosphorylation) into PLK4 (top panel) and lacks detectable T170PLK4 phosphorylation before or after ATP addition when probed with pT170PLK4 PLK4 antibody (middle panel). Equal loading of proteins was confirmed by staining the membrane with Ponceau S (bottom panel).
