Figure 6.
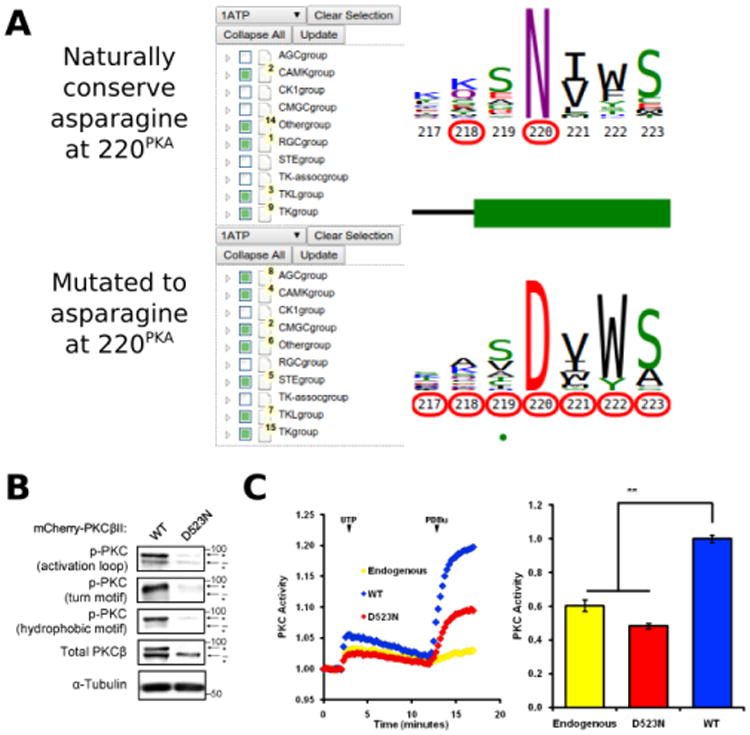
A) KinView selection of kinases that naturally conserve an asparagine at position 220PKA (top) and those that have mutations to asparagine at position 220PKA (bottom). Note that the AGC group does not naturally conserve an asparagine at 220PKA among the 15 organisms included in ProKinO. B) Impaired phosphorylation of D523NPKCβ PKCβII variant. The mutation to asparagine decreases the priming phosphorylations, which require the activity of PKC, in the activation loop, the C-tail turn motif and the C-tail hydrophobic motif. C) Normalized FRET ratio changes showing PKC activity from COS7 cells co-expressing CKAR and mCherry-tagged PKCβII mutant stimulated with 100μM UTP followed by 200 nM PDBu. Graph on the right shows the signaling output resulting from UTP stimulation (see Methods), quantified and normalized to WT PKC activity. **p<0.01 as compared with WT, using a repeated-measures one-way ANOVA.
