Abstract.
Recent studies have demonstrated an association between congenital Zika virus (ZIKV) infection and microcephaly; however, to date, there have been no reports on the consequences of ZIKV infection on fetuses in twin pregnancies. Herein, we reported on the first case of a monochorionic diamniotic (MCDA) twin pregnancy having ZIKV-related microcephaly. Our findings suggested that, in an MCDA twin pregnancy, the ZIKV may cause infection in both fetuses, resulting in severe abnormalities in the central nervous system due to neural cell destruction and the disruption of the normal development processes of the brain. This case report and other similar twin cases may help to understand the pathogenesis and to confirm the etiology of ZIKV as a teratogenic microorganism.
INTRODUCTION
The association of the Zika virus (ZIKV) with congenital malformations emerged as another significant global public health problem. In Brazil, an epidemic of ZIKV has been reported since 2015, coinciding with an unusual increase in Guillain–Barré syndrome and microcephaly.1 Increasing evidence suggests that ZIKV may induce neurological disorders, ocular abnormalities, and death in fetuses from mothers infected at different stages of their pregnancy.2–4 However, to date, there is no report of congenital ZIKV infection in a twin pregnancy. In this report, a case of twins having ZIKV-related microcephaly in the Sergipe State (in the northeast of Brazil) is presented, and the main findings and pathogenic aspects of this case are discussed.
CASE REPORT
A 17-year-old primigravida sought assistance for prenatal care at the public service unit at 8 weeks of pregnancy for prenatal care and a monochorionic diamniotic (MCDA) twin pregnancy was diagnosed by ultrasound. She reported no significant medical or family history and no exposure to medications, radiation, or infection. At the 33rd week of pregnancy, one twin was suspected of having a small head circumference for its gestational age by fetal ultrasound. Pre- and postnatal serological tests ruled out toxoplasmosis, rubella, cytomegalovirus, herpes simplex, syphilis, and human immunodeficiency virus infections.
At birth, both twins had small head circumferences compared with the mean for sex (female) and gestational age (34th week) in the Fenton growth chart5 (twin 1: 26.0 cm and twin 2: 28.5 cm) (Table 1). Transfontanellar ultrasonography showed that both neonates presented suggestive signs of subependymal hemorrhage, multiple subcortical punctiform calcifications, reduced corpus callosum area and cerebral parenchyma, and reduced cortical thickness. Twin 1 had enlarged atria and occipital horns of the lateral ventricles. An enlargement of the subarachnoid space was observed in twin 2 (Figures 1 and 2). Serum samples from the mother and the twins were tested for IgM and IgG antibodies specific for dengue, chikungunya, and yellow fever and they were negatives. In addition, serum samples from the mother and the twins were tested for ZIKV-specific IgM antibodies using a capture enzyme-linked immunosorbent assay and by quantitative reverse transcription polymerase chain reaction (RT-PCR) based on the U.S. Centers for Disease Control and Prevention protocol. The ZIKV serological test was positive for the mother, but not for the twins, whereas the RT-PCR in serum was ZIKV negative for the mother and both twins. These findings fulfill the Brazilian Ministry of Health’s criteria for Zika-related microcephaly.6
Table 1.
Summary of the main clinical, ophthalmic, hearing, and radiologic findings
| Findings | Twin 1 | Twin 2 |
|---|---|---|
| Clinical | ||
| Sex | Female | Female |
| Head circumference at birth (cm) | 26.0 | 28.5 |
| RT-PCR or Zika virus-specific IgM | Negative | Negative |
| Ophthalmological | ||
| Exotropia | Yes | Yes |
| Nystagmus | Yes | No |
| Focal pigment mottling | No | No |
| Uveitis | No | No |
| Retina and optic nerve abnormalities | No | No |
| Hearing screening | ||
| Hearing loss | Yes | No |
| Transfontanellar ultrasonography | ||
| Subependymal hemorrhage | Yes | Yes |
| Multiple punctiform calcifications | Yes | Yes |
| Reduced corpus callosum and cerebral parenchyma, and cortical thickness | Yes | Yes |
| Enlargement of atria and occipital horns of the lateral ventricles | Yes | No |
| Enlargement of the subarachnoid space | No | Yes |
RT-PCR = reverse transcription polymerase chain reaction.
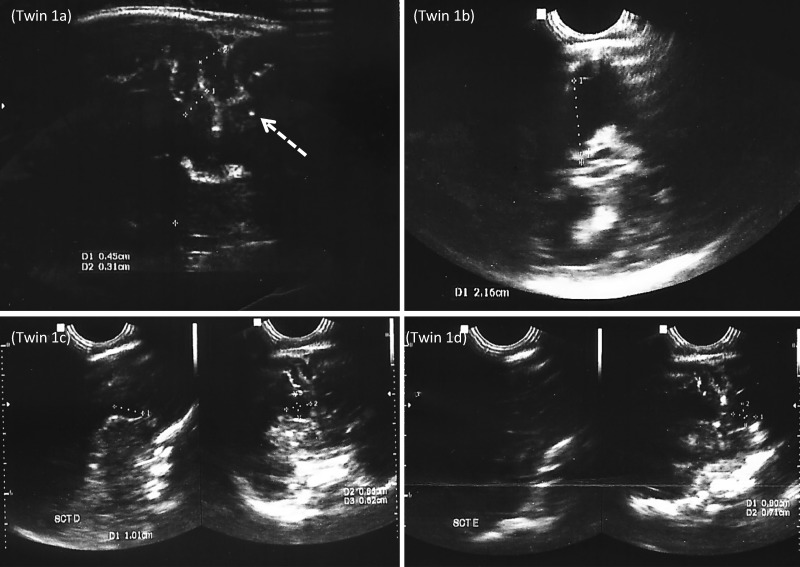
Figure 1.
Twin 1: (A) Coronal plane showing a decreased cortical thickness, increased ventricular size, and intracranial calcification (arrow). (B) Axial image showing enlargement of atria and occipital horns of the lateral ventricles. (C and D) Sagittal and coronal ultrasonography demonstrating bilateral subependymal cysts in the caudothalamic groove.
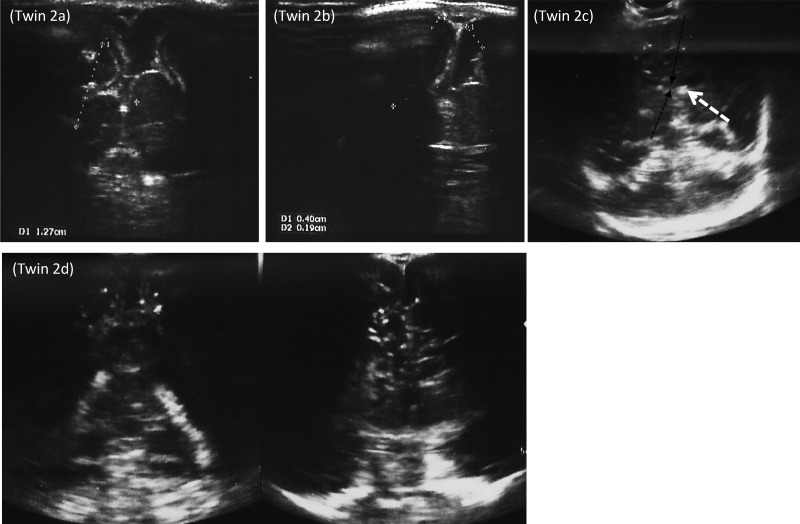
Figure 2.
Twin 2: Coronal planes showing (A) decrease in the cortical thickness, (B) enlargement of the subarachnoid space, (C) reduced corpus callosum (black arrow) and a calcification in the lentiform nucleus (white arrow), and (D) multiple intracranial calcifications and subependymal cysts.
On ophthalmologic examination, both neonates had exotropia (twin 1: 25Δ and twin 2: 45Δ). Twin 1 showed poor fixation and horizontal left beating nystagmus. Slit-lamp, pupillary, and fundus examinations were normal for both the twins. In addition, the neonates underwent a newborn hearing screening by otoacoustic emission and brain evoked response audiometry tests according to Brazilian Ministry of Health protocol (http://portalsaude.saude.gov.br/index.php/cidadao/principal/agencia-saude/21241-saude-lanca-protocolo-de-atencao-a-saude-para-microcefalia). Twin 1 exhibited an interaural difference of wave V above 0.3 ms, suggesting retrocochlear pathology, whereas twin 2 showed no alteration (Table 1).
DISCUSSION
Recent studies have demonstrated an association between congenital ZIKV infection and microcephaly.7 However, there have been no reports on the consequences of ZIKV infection on fetuses in twin pregnancies. Herein, we report on the first case of MCDA twins having ZIKV-related microcephaly with neurological disorders and ocular and hearing abnormalities.
ZIKV apparently exhibits neural tissues tropism, affecting cells growth during fetal life. An in vitro study suggests that early ZIKV infection induces cell death in human neural stem cells, disrupting the formation of neurospheres, and reducing the growth of organoids.8 A Brazilian ZIKV strain infecting pregnant mice caused deregulation of the genes related to autophagy and apoptosis, resulting in intrauterine brain growth restriction.9
Ocular lesions in infants with ZIKV-related microcephaly have also been described.10 Common ophthalmologic findings include focal pigment mottling, chorioretinal atrophy, and optic nerve abnormalities.10,11 Isolated case of uveitis also have been reported.12 In our study, no eye fundus lesions were observed. However, one of the twins had horizontal left beating nystagmus, a condition that has not previously been reported in infants having microcephaly due to ZIKV infection. Horizontal nystagmus usually results from lesions involving the paramedian pontine reticular formation.13–15 Further studies are needed to investigate whether there is an association between ZIKV infection and nystagmus.
The incidence of hearing loss in children with congenital ZIKV infection is unknown. Recently, sensorineural hearing loss was documented in four of 69 infants (5.8%) with microcephaly and laboratory evidence of congenital ZIKV infection.16 In this case report, one of the twins presented hearing loss suggestive of retrocochlear pathology, similar to abnormalities caused by other viruses associated with microcephaly and congenital hearing loss, such as cytomegalovirus, rubella, lymphocytic choriomeningitis virus, and herpes simplex virus.17 As it is not known whether the onset of hearing loss and ophthalmologic lesions associated with congenital ZIKV infection could be delayed in the other twin, whether the ZIKV may have infected the twins at different times during pregnancy, or whether the expression of congenital infection may be different even in MCDA twins, we recommend to repeat the hearing evaluation at 6 months of age.18 This has been described respect to other infections, such as cytomegalovirus.19 However, further studies are needed to establish whether hearing loss is due to the direct effects of ZIKV on the inner ear structures, host immune responses to viral antigens in the cochlear area, or whether there is a concomitant ZIKV infection in the central auditory brain area.
Studies have recently shown that ZIKV infects human placental tissue, particularly placental macrophages/Hofbauer cells (HBCs).20–22 As the HBCs are located beneath the syncytium and adjacent to fetal capillaries, a critical site for protection of the fetus against infectious agent migrants from mother to the fetus, it is possible that the HBCs infection plays an important role in the vertical transmission of ZIKA.23 The infection of HBCs is typically present in most maternal blood-borne infections that are vertically transmitted, such as toxoplasmosis, syphilis, rubella, cytomegalovirus, and herpes simplex virus.24,25 However, further studies are needed to elucidate the specific role of HBCs in the spread of the ZIKV.
Although genetically identical fetuses provide an important model for discussion of diseases on the same maternal influence, studies on other viruses have shown that twin fetuses may react differently to primary maternal infection due to the virus load and placental characteristics.26 Studies on cytomegalovirus have shown that the risk of infection of both fetuses is higher in monochorionic pregnancies than in dichorionic pregnancies; and the presence of separate placentas does not eliminate the possibility that both twins will be infected.26–28 In this case report, despite both twins having exhibited microcephaly and important abnormalities, only one twin presented hearing loss and ophthalmologic alterations. Unfortunately, it was not possible to establish if there were marked differences in the quantity of virus load in the prenatal period.
In summary, our findings suggest that, in an MCDA twin pregnancy, the ZIKV may cause infection in both fetuses, resulting in severe abnormalities in the central nervous system including brain dysgenesis and intracranial calcifications. However, the ZIKV may cause outcomes with different degrees of severity in each of the fetuses. Further studies are needed to answer important questions: 1) is it possible that the ZIKV can infect only one twin of a pair? 2) has twin–twin transmission been proposed in instances where there would likely be two placentas? and 3) what is the role of the placenta, viral tropism, and immunogenetic factors that might contribute to the susceptibility of twins to ZIKV infection? This case report and other similar twin cases may help in understanding the pathogenesis and in confirming the etiology of ZIKV as a teratogenic microorganism.
REFERENCES
- 1.Victora CG, Schuler-Faccini L, Matijasevich A, Ribeiro E, Pessoa A, Barros FC, 2016. Microcephaly in Brazil: how to interpret reported numbers? Lancet 387: 621–624. [DOI] [PubMed] [Google Scholar]
- 2.de Noronha L, Zanluca C, Azevedo MLV, Luz KG, dos Santos CND, 2016. Zika virus damages the human placental barrier and presents marked fetal neurotropism. Mem Inst Oswaldo Cruz 111: 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatnagar J, Rabeneck DB, Martines RB, Reagan-Steiner S, Ermias Y, Estetter LBC, Suzuki T, Ritter J, Keating MK, Hale G, Gary J, Muehlenbachs A, Lambert A, Lanciotti R, Oduyebo T, Meaney-Delman D, Bolaños F, Saad EAP, Shieh W-J, Zaki SR, 2017. Zika Virus RNA replication and persistence in brain and placental tissue. Emerg Infect Dis 23: 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR, 2016. Zika virus and birth defects: reviewing the evidence for causality. N Engl J Med 374: 1981–1987. [DOI] [PubMed] [Google Scholar]
- 5.Fenton TR, Kim JH, 2013. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 13: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brazil, Ministério da Saúde , 2016. Informe Epidemiológico N° 31—Semana Epidemiológica (SE) 24/2016 (12 a 18/06/2016) Monitoramento dos casos de microcefalia no Brasil. Available at: http://combateaedes.saude.gov.br/images/pdf/informe_microcefalia_epidemiologico31.pdf. Accessed January 7, 2017.
- 7.de Araujo TVB, Rodrigues LC, de Alencar Ximenes RA, de Barros Miranda-Filho D, Montarroyos UR, de Melo APL, Valongueiro S, de Albuquerque M de FPM, Souza WV, Braga C, Filho SPB, Cordeiro MT, Vazquez E, Di Cavalcanti Souza Cruz D, Henriques CMP, Bezerra LCA, da Silva Castanha PM, Dhalia R, Marques-Junior ETA, Martelli CMT; Investigators from the Microcephaly Epidemic Research Group; Brazilian Ministry of Health; Pan American Health Organization; Instituto de Medicina Integral Professor Fernando Figueira; State Health Department of Pernambuco, 2016. Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: preliminary report of a case-control study. Lancet Infect Dis 3099: 1–8. [DOI] [PubMed] [Google Scholar]
- 8.Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, Rehen SK, 2016. Zika virus impairs growth in human neurospheres and brain organoids. Science 352: 816–818. [DOI] [PubMed] [Google Scholar]
- 9.Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JLM, Guimarães KP, Benazzato C, Almeida N, Pignatari GC, Romero S, Polonio CM, Cunha I, Freitas CL, Brandão WN, Rossato C, Andrade DG, Faria D de P, Garcez AT, Buchpigel CA, Braconi CT, Mendes E, Sall AA, Zanotto PMDA, Peron JPS, Muotri AR, Beltrão-Braga PCB, 2016. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534: 267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Paula Freitas B, de Oliveira Dias JRJ, Prazeres J, Sacramento GAG, Ko AI, Maia MM, Belfort RJ, 2016. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital. JAMA Ophthalmol 134: 529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ventura CV, Maia M, Travassos SB, Martins TT, Patriota F, Nunes ME, Agra C, Torres VL, van der Linden V, Ramos RC, Rocha MÂW, Silva PS, Ventura LO, Belfort R, 2016. Risk factors associated with the ophthalmoscopic findings identified in infants with presumed Zika virus congenital infection. JAMA Ophthalmol 134: 912–918. [DOI] [PubMed] [Google Scholar]
- 12.Kodati S, Palmore TN, Spellman FA, Cunningham D, Weistrop B, Sen HN, 2016. Bilateral posterior uveitis associated with Zika virus infection. Lancet 6736: 10–11. [DOI] [PubMed] [Google Scholar]
- 13.Bae YJ, Kim JH, Choi BS, Jung C, Kim E, 2013. Brainstem pathways for horizontal eye movement: pathologic correlation with MR imaging. Radiographics 33: 47–59. [DOI] [PubMed] [Google Scholar]
- 14.Strupp M, Hüfner K, Sandmann R, Zwergal A, Dieterich M, Jahn K, Brandt T, 2011. Central oculomotor disturbances and nystagmus: a window into the brainstem and cerebellum. Dtsch Arztebl Int 108: 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Büttner U, Büttner-Ennever JA, 2006. Present concepts of oculomotor organization. Prog Brain Res 151: 1–42. [DOI] [PubMed] [Google Scholar]
- 16.Leal MC, Muniz LF, Ferreira TSA, Santos CM, Almeida LC, Van Der Linden V, Ramos RCF, Rodrigues LC, Neto SSC, 2016. Hearing loss in infants with microcephaly and evidence of congenital Zika virus infection: Brazil, November 2015–May 2016. MMWR Morb Mortal Wkly Rep 65: 917–919. [DOI] [PubMed] [Google Scholar]
- 17.Cohen BE, Durstenfeld A, Roehm PC, 2014. Viral causes of hearing loss: a review for hearing health professionals. Trends Hear 18: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell K, Oliver SE, Lewis L, Barfield WD, Cragan J, Meaney-Delman D, Staples JE, Fischer M, Peacock G, Oduyebo T, Petersen EE, Zaki S, Moore CA, Rasmussen SA, 2016. Update: interim guidance for the evaluation and management of infants with possible congenital Zika virus infection: United States, August 2016. MMWR Morb Mortal Wkly Rep 65: 870–878. [DOI] [PubMed] [Google Scholar]
- 19.Dahle AJ, Fowler KB, Wright JD, Boppana SB, Britt WJ, Pass RF, 2000. Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J Am Acad Audiol 11: 283–290. [PubMed] [Google Scholar]
- 20.Jurado KA, Simoni MK, Tang Z, Uraki R, Hwang J, Householder S, Wu M, Lindenbach BD, Abrahams VM, Guller S, Fikrig E, 2016. Zika virus productively infects primary human placenta-specific macrophages. JCI Insight 1: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O’Neal JT, Rajakumar A, Wrammert J, Rimawi BH, Pulendran B, Schinazi RF, Chakraborty R, Suthar MS, 2016. Zika virus infects human placental macrophages. Cell Host Microbe 20: 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg AZ, Yu W, Hill DA, Reyes CA, Schwartz DA, 2016. Placental pathology of Zika virus: viral infection of the placenta induces villous stromal macrophage (Hofbauer cell) proliferation and hyperplasia. Arch Pathol Lab Med 141: 43–48. [DOI] [PubMed] [Google Scholar]
- 23.Simoni MK, Jurado KA, Abrahams VM, Fikrig E, Guller S, 2016. Zika virus infection of Hofbauer cells. Am J Reprod Immunol 77: e12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nahmias AJ, Panigel M, Schwarts DA, 1994. The eight most frequent blood-borne infectious agents affecting the placenta and fetus: a synoptic review. Trophob Research 8: 193–213. [Google Scholar]
- 25.Satosar A, 2004. Histologic correlates of viral and bacterial infection of the placenta associated with severe morbidity and mortality in the newborn. Hum Pathol 35: 536–545. [DOI] [PubMed] [Google Scholar]
- 26.Lazzarotto T, Gabrielli L, Foschini MP, Lanari M, Guerra B, Eusebi V, Landini MP, 2003. Congenital cytomegalovirus infection in twin pregnancies: viral load in the amniotic fluid and pregnancy outcome. Pediatrics 112: 133–137. [DOI] [PubMed] [Google Scholar]
- 27.Egaña-Ugrinovic G, Goncé A, García L, Marcos MA, López M, Nadal A, Figueras F, 2015. Congenital cytomegalovirus infection among twin pairs. J Matern Neonatal Med 29: 3439–3444. [DOI] [PubMed] [Google Scholar]
- 28.Yinon Y, Yagel S, Tepperberg-Dikawa M, Feldman B, Schiff E, Lipitz S, 2006. Prenatal diagnosis and outcome of congenital cytomegalovirus infection in twin pregnancies. BJOG An Int J Obstet Gynaecol 113: 295–300. [DOI] [PubMed] [Google Scholar]