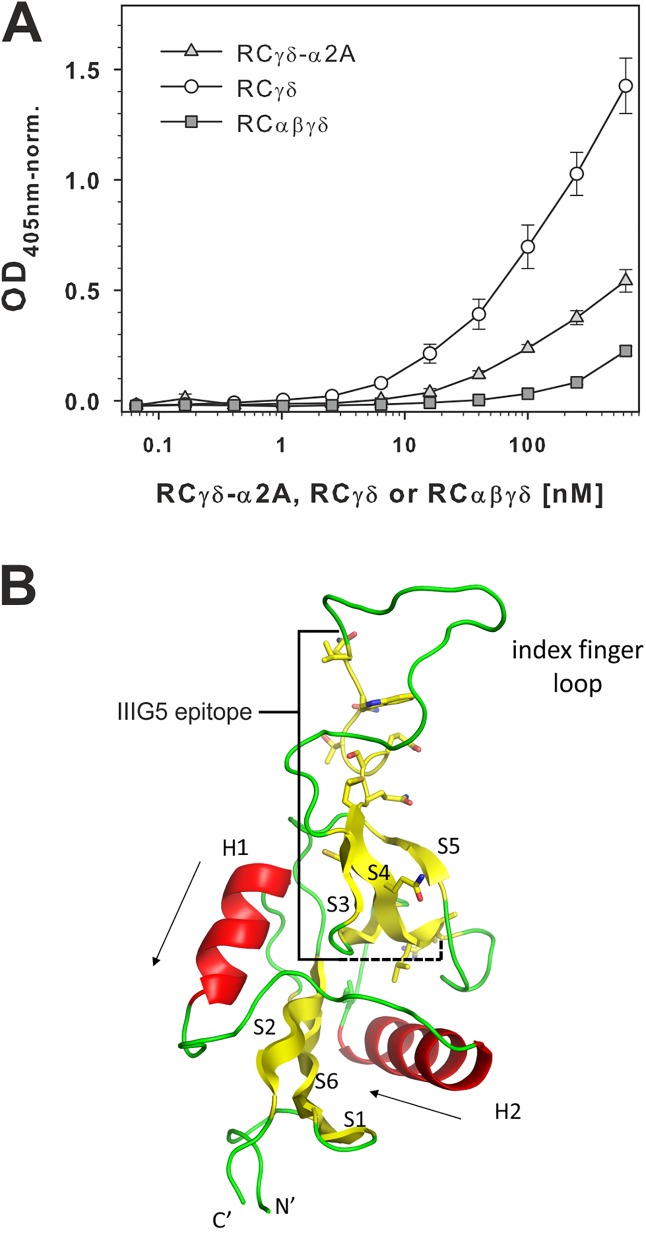
Fig 4. The monoclonal antibody IIIG5 recognizes its epitope within the RCγ subunit in the RCγδ-α2A complex but not in the tetrameric RCαβγδ.
(A) The monoclonal antibody IIIG5 recognized an epitope of the RCγ subunit, which is fully accessible in the RCγδ subunit (○), partially accessible in the RCγδ-α2A complex (light gray ▲), and completely covered in the RCαβγδ tetramer (dark gray ■). IIIG5 was immobilized on microtiter plates and titrated with RCαβγδ, RCγδ-α2A complex, or RCγδ subunit. Bound rhodocetin (RC) components were fixed and detected using rabbit RC antiserum with ELISA at 405 nm. The data presented here are taken from 3 independent experiments with each measurement done in duplicate. Means ± SD are shown. The data are summarized in S1 Data. (B) Molecular structure of the C-type lectin-related protein (CLRP)-fold typical of all 4 RC chains. Both the γ and δ subunits of RC are very similar (Cα-RMSD 0.8Å) and feature a core structure with 2 α-helices (H1 and H2) flanked by 2 antiparallel β-sheets (S1–S2–S6 and S3–S4–S5). The amino acid residues V94–R109 of the IIIG5 epitope of RCγ are highlighted.