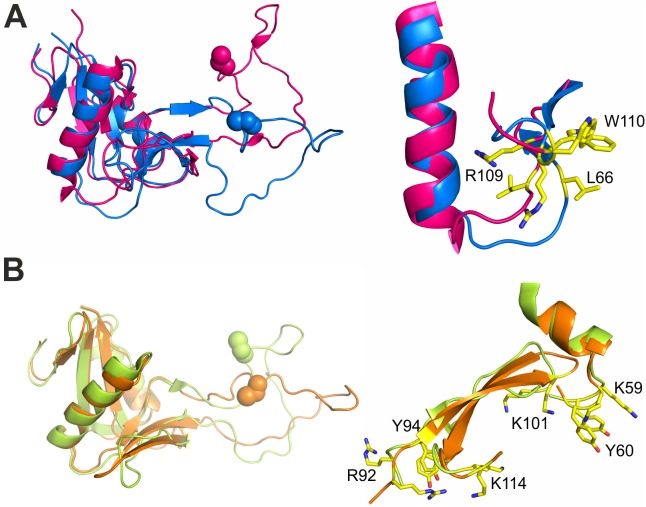
Fig 6. An overview of the RCγ and RCδ binding residues, depicting the local conformational changes that occur upon α2A binding.
(A) A comparison of the RCγ subunit binding site (L66/R109/W110) between the RCαβγδ (purple) and RCγδ-α2A complex (blue) structures. Due to the global movements within the index finger swapping domain that accompany the formation of the RCγδ-α2A complex, a local repositioning of the key α2A interacting residues within RCγ takes place such that they adopt an orientation that is compatible for α2A binding. (B) A comparison between the 2 RCδ subunit binding sites (K59/Y60/K101 and R92/Y94/K114) between the RCαβγδ (yellow) and RCγδ-α2A complex (orange) structures. In contrast to RCγ, all the RCδ residues involved in α2A binding would be in an α2A-competent orientation in both the RCαβγδ (yellow) and RCγδ-α2A complex (orange) structures, with the exception of R92, which forms an internal salt bridge with D74γ in the RCαβγδ tetramer but interacts with D219 of α2A in the RCγδ-α2A complex.