Abstract
Main objective
To better understand the role of myeloperoxidases (MPO) in microvascular obstruction (MO) phenomenon and infarct size (IS) using cardiac magnetic resonance (CMR) data in patients with acute myocardial infarction (AMI).
Method
40 consecutive patients classified according to the median level of MPO in the culprit artery. A CMR study was performed during the week following AMI and at 6 months, with late gadolinium enhancement sequences.
Results
Persistent MO was observed in the same proportion (50 vs. 65%, p = 0.728) between the low vs. high MPO group levels. However, the extent of the microvascular obstruction was significantly greater in the high-MPO group (6 (0–9) vs.16.5 (0–31), p = 0.027), together with a greater infarct size, and a trend towards a lower left ventricular ejection fraction (LVEF) (p = 0.054) at one week. CMR data at 6 months showed that reverse systolic remodeling was two fold more present in the low-MPO group (p = 0.058). Interestingly, the extent of MO (8.5 (6.5–31) vs. 4.1 (3–11.55), p = 0.042) and IS remained significantly greater (24.5 (9.75–35) vs. 7.5 (2.5–18.75), p = 0.022) in the high-MPO group. Moreover, MPO in the culprit artery appeared to correlate positively with MPO in non-culprit arteries and serum, and with troponin levels and peak CK.
Conclusion
This patient-based study revealed in patients after AMI that high MPO levels in the culprit artery were associated with more severe microvascular obstruction and greater IS. These findings may provide new insights pathophysiology explanation for the adverse prognostic impact of MO.
Introduction
Myeloperoxidase (MPO) is a member of the heme peroxidase superfamily. It is secreted from activated neutrophils, monocytes and certain tissue macrophages, and generates reactive oxygen species and diffusible radical species [1]. Studies have shown that MPO levels are a predictor of death after chest pain [2] and acute myocardial infarction [3]. However, the mechanisms by which MPO adversely affects the prognosis are only partly understood. In addition to its pathophysiological role in atherogenesis [4], MPO has recently been shown to contribute to microvascular obstruction in AMI patients [5]. During myocardial ischemia-reperfusion sequences, microvascular function is associated with recruitment of polymorphonuclear neutrophils (PMN) and has been attributed to decreased bioavailability of nitric oxide (NO). Endothelium-dependent microvascular function is a multifactorial process involving endothelial injury or dysfunction, neutrophil accumulation, the over-production of reactive oxygen species, thrombus embolization and activation of the coagulation cascade [6]. Among different tools used to assess the microvascular obstruction, cardiac magnetic resonance imaging (CMR) with a gadolinium-based contrast agent shows the extent of myocardial damage following infarction, areas of hyperenhancement on late gadolinium-enhanced (LGE) images (= infarct size) and left ventricular remodeling indexes [7]. Moreover, regions of persistent hypoenhancement in the core of the infarcted myocardium have been shown to reflect no reflow [8]. One of the most important pathogenic pathways involving MPO might be endothelial dysfunction. Considering the strong relation between MPO and systemic endothelial function, it might also be speculated that systemic activation of leukocytes, particularly via secretion of MPO, is one mechanism by which AMI leads to systemic endothelial dysfunction [9]. It has been suggested that both endothelial function and LDL/HDL oxidation due to MPO release are involved in the occurrence of NR and its consequences such as infarct size (IS) enlargement and left ventricular (LV) remodeling. The objectives of our study were to assess plasma MPO levels, and endothelial dysfunction witnessed by plasma levels of L-arginine and its methylated derivatives, asymmetric and symmetric dimethylarginine (ADMA and SDMA) at admission in first ST elevation myocardial infarction (STEMI) patients, and to explore the relationship between such biomarkers with microvascular obstruction, IS and LV remodeling by cardiac magnetic resonance (CMR) at 3 days and 6 months.
Patients and methods
The participants in this study were recruited from the observatoiRe des Infarctus de Côte d’Or (RICO) survey, a French regional survey for AMI, the details of which have been previously published [10]. In the present study, we design a patient-based study including male patients admitted with a first acute ST-Elevation MI within 12 hours after symptom onset were included. MI was diagnosed according to European Society of Cardiology and American College of Cardiology criteria [11]. All patients were treated by a primary or rescue PCI procedure with thrombectomy. Patients under 18 years of age, patients with cardiogenic shock, acute infection, acute inflammation, severe renal impairment, or with a prior chronic treatment (or for at least 7 days) with vitamin C, steroids or non-steroid anti-inflammatory drugs, and those with a CRP> 30 mg/l were excluded from the study. Forty-nine patients with AMI were recruited during the study period. Five were excluded because of a contra-indication to CMR (mainly claustrophobia), and 4 because of incomplete biological data. This study complied with the Declaration of Helsinki, was approved by the ethics committee of the University Hospital of Dijon, and each patient gave written consent before participation.
Data collection
The data included demographics, cardiovascular risk factors (history of hypertension or treated hypertension, diabetes, history of hypercholesterolemia or treated hypercholesterolemia, current smoking), admission characteristics and hemodynamic parameters. Patients’ BMI (weight (kg)/height (m2)) was measured within 48 hours of admission. Ischemic time was the time between symptom onset and crossing the culprit lesion with the floppy guide wire. We recorded anterior wall infarction, and coronary angiograms before intervention, after aspiration thrombectomy, and at the end of the procedure were analyzed. We evaluated baseline, post-thrombectomy, and final antegrade coronary flow according to the TIMI (Thrombolysis In Myocardial Infarction) criteria, together with the final myocardial blush grades. No reflow was defined as TIMI flow grade ≤2 or TIMI flow grade 3 with myocardial blush grade ≤1 at the final angiogram [12]. Blood samples were drawn at admission. We assessed peak plasma CK by sampling every eight hours during the first two days after admission. Plasma creatinine levels were measured on a Vitros 950 analyzer (Ortho Clinical Diagnostics, Rochester, NY). C-reactive protein (CRP) was measured on Dimension Xpand (Dade Behring, Newark, Neb) with an immunonephelometry assay. Plasma N-terminal pro B-type natriuretic peptide (NT-proBNP) was determined by ELISA with an Elecsys NT-proBNP sandwich immunoassay on Elecsys 2010 (Roche Diagnostics, Basel, Switzerland). Total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and triglyceride (TG) concentrations were measured on a Dimension analyzer (Dade Behring, Newark, NE). The level of low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald formula. Plasma glucose concentrations (enzymatic method (glucose oxidase)) and creatinine levels were measured on a Vitros 950 analyzer (Ortho Clinical Diagnostics, Rochester, NY) and creatinine clearance was calculated with the Cockcroft formula. Glycated hemoglobin A1c (HbA1c) was measured with ion exchange HPLC (Bio-Rad Laboratories, Richmond, CA).
Oxidative stress, endothelial function and inflammation markers
Plasma myeloperoxidase levels, reactive oxygen species (ROS) assay, plasma antioxidant activity, L-arginine, ADMA and SDMA were determined at 3 sites: 1) venous blood samples and other blood samples taken before angioplasty and stenting from the 2) coronary ostium of a non-culprit artery through the guiding catheter and from 3) the culprit artery at the culprit lesion site by the Export® thrombectomy catheter. It consisted of a 6-Fr rapid exchange catheter (1.37 mm internal lumen) and vacuum syringe connected to its proximal end. The distal tip was advanced along the guide wire up to the thrombus and then the vacuum syringe was opened to aspirate the thrombus from the coronary artery. Samples were centrifuged at 2000 x g for 10 minutes at 4°C. The supernatants were decanted and frozen at -80°C until analysis.
Plasma MPO concentrations were measured by quantitative sandwich enzyme immunoassay (Human MPO, Quantikine® ELISA, R&D Systems, Abingdon, UK). ROS were determined using the free oxygen radical monitor (FORM, FORM-PLUS-3000, Optimabio, France) and kit. The FORM indirectly measures the concentration of hydroperoxides by a colorimetric assay using Free Oxygen Radical Testing (FORT) as previously described [13]. The ROS concentration is reported in FORT units, where 1 FORT unit corresponds to 7.6μmol/L (0.26 mg/L of H2O2). Total plasma Oxygen Radical Antioxidant Capacity (ORAC) was determined by the method first described by Cao et al. as detailed [14]. The method is based on the ability of plasma components to protect an indicator protein, allophycocyanin (APC), whose fluorescence is altered when it is oxidized. The results are expressed as ORAC units, where 1 ORAC unit equals the net protection provided by 1 μmol/L Trolox.
L-arginine, ADMA, and SDMA were measured by high performance liquid chromatography (HPLC) as previously described [15]. The detection limits were 0.05 and 1.19 μmol/L and inter-day variabilities were 5.7 and 4.6% for ADMA and L-arginine, respectively.
CMR protocol
Patients underwent CMR a mean of 3 days (3±2 days) after admission and 6 months after AMI. CMR was performed on a 3.0-T Magnet (Trio TIM; Siemens Medical Solutions, Germany), using a phased-array thoracic coil. Patients who did not benefited from two CMRI examinations were excluded from the study. In all sequences, the signal intensity correction algorithm provided by the manufacturer was applied. The patient was placed in the supine position and images were acquired under cardiac gating. To evaluate left ventricular function, cine-CMR was performed using a breath-hold, ECG-gated trueFISP sequence, (TR/TE 3.4/1.7 ms, FA 57°, FOV 350x420 mm2, acquisition matrix 150x192). A series of short-axis slices (thickness 5 mm, interslice gap 5 mm) was defined from the base of the heart to the apex. To evaluate microvascular obstruction and infarct size, Late Gadolinium-Enhanced (LGE) images were acquired 10 minutes after a bolus injection of 0.1 mmol/Kg Gd-DOTA into a brachial vein. A segmented T1-weighted Phase-Sensitive Inversion Recovery (PSIR) sequence was used (TR/TE/TI: 3.5/1.42/400 ms, FA 20°, matrix 175x256, FOV 240x350 mm2, pixel size 1.37x1.37 mm2; acquisition time of 10 to 15 seconds according to the RR; cardiac gating every second cycle). The image planes were the same as for the functional and perfusion studies (thickness of 8 mm with an interslice gap of 2 mm). Horizontal and vertical long-axis slices were also obtained. The sequence parameters were: repetition time/echo time = 780/1.56 ms, flip angle = 10, field of view = 350x400 cm, and matrix = 128x256, in plane resolution = 2.3x1.2. The inversion time was 270–325 ms (set to null normal myocardium). The end-diastolic phase was chosen to set the acquisition window. The images at the end-systole and end-diastole were taken into account.
CMR data analysis
Prospective image analyses were performed using QIR® software (Université de Bourgogne, Dijon, France). Left ventricular End-Diastolic Volume (EDV) and End-Systolic Volume (ESV) were calculated from short-axis views. The Left Ventricular Ejection Fraction (LVEF) was calculated with the following formula: LVEF = (EDV-ESV)/EDV. Reverse remodeling was defined as a decrease in LVESV >10% on the cardiac MR images obtained one week and 6 months after STEMI [16]. For the detection and quantification of the infarct zone and microvascular obstruction, an automatic method was used. First, the endocardial and epicardial contours were manually traced on each short-axis LGE image. The myocardium area was segmented as normal and pathological areas using a Gaussian mixture model (GMM).
Microvascular obstruction was defined as a dark area surrounded by the MI area. Infarct size (IS) was determined for each patient by summing the results for each slice multiplied by the gap between slices, and was expressed as a percentage of total myocardial volume. In addition, delayed enhancement images were visually analyzed according to a 17-segment model. Images were analyzed by the consensus of 2 expert observers, blinded to patient data and the results of other examinations. The inter-observer variability was less than 5%. In cases of disagreement, the final decision was taken by consensus.
Statistical analysis
Patients were dichotomized into two groups according the median MPO levels in the culprit artery (median value = 640 ng/mL.). The results are expressed as median values (25th-75th percentile) for continuous variables or as percentages for qualitative variables. The normality of distribution for each variable was analyzed by the Kolmogorov-Smirnov test. The Spearman or Pearson test was used to determine correlations between continuous variables and MPO values. For binary variables, the median MPO levels were compared with a Mann-Whitney test. A multiple linear regression analysis was performed to determine the independent factors associated with MPO levels. All variables listed in Table 1 were tested in univariate analysis and were introduced into the multivariate model if the p value<0.20. All analyses were performed using Sigmastat Software (Jandel Inc.).
Table 1. Patients’ characteristics.
Data are presented as n (%) or median (25th-75th).
| Low MPO N = 20 |
High MPO N = 20 |
p | |
|---|---|---|---|
| Age, years | 59(48–66) | 54(48–66) | 0.766 |
| BMI, kg/m2 | 25.50(23.25–28.00) | 26(24.25–28.00) | 0.599 |
| Waist circumference, cm | 96(86.25–103) | 96.50(90.00–103.25) | 0.841 |
| Hypertension, | 5(25) | 4(20) | 0.705 |
| Hypercholesterolemia | 8(40) | 4(20) | 0.168 |
| Family history of CAD | 1(5) | 0 | 0.311 |
| Diabetes | 0 | 2(10) | 0.147 |
| Smoking | 13(65) | 9(45) | 0.204 |
| Clinical data | |||
| SBP, mmHg | 132.5(118.0–147.5) | 128.0(116.5–148.0) | 0.903 |
| LVEF, % | 55(46–60) | 53(36–60) | 0.178 |
| LVEF>40% | 18(56) | 14(43) | 0.114 |
| GRACE risk score | 82(60–104) | 74(65–106) | 0.924 |
| Delay>120 min | 5(45.5) | 6(54.5) | 0.383 |
| Previous angina | 13(46) | 15(54) | 0.490 |
| KILLIP adm>1 | 1(25) | 3(75) | 0.292 |
| Anterior wall location | 8(40) | 13(65) | 0.113 |
| Thrombolysis | 7(34) | 3(16) | 0.200 |
| PPCI | 12(60) | 14(78) | 0.239 |
| Delay symptoms-reperfusion, min | 190(112.5–360) | 180(115–355) | 0.957 |
| STR>50% | 16(94) | 15(83) | 0.316 |
| Post-PCI No-reflow in angio TIMI | 1(5) | 3(16) | 0.267 |
| Biological data | |||
| HDL, mmol/L | 0.38(0.35–0.41) | 0.47(0.40–0.56) | 0.017 |
| LDL, mmol/L | 1.44(1.15–1.60) | 1.58(1.19–1.77) | 0.267 |
| TC, mmol/L | 2.23(1.83–2.35) | 2.24(1.94–2.47) | 0.423 |
| Log CK | 3.10(2.73–3.42) | 3.60(3.36–3.74) | 0.003 |
| Log BNP | 1.85(1.67–2.47) | 2.05(1.73–2.83) | 0.308 |
| Glycemia, mmol/L | 6.85(6.31–8.17) | 7.13(6.14–8.44) | 0.803 |
| HbA1C, % | 5.70(5.50–6.00) | 5.95(5.53–6.23) | 0.181 |
| CRP, mg/L | 3.95(2.99–11.25 ) |
3.00(2.92–5.75) | 0.545 |
| Creat cl, mL/min | 104(78–122) | 98(77–120) | 0.963 |
| MPO nc, ng/ml | 494.23(380.76–658.59) | 810.25(672.54–997.59) | 0.001 |
| MPO vn, ng/ml | 418.02(302.50–546.54) | 769.82(425.74–1075.22) | 0.003 |
| ADMA ca, μmol/L | 0.31(0.26–0.38) | 0.34(0.28–0.41) | 0.552 |
| ADMA nc, μmol/L | 0.35(0.30–0.43) | 0.37(0.30–0.45) | 0.818 |
| ADMA vn, μmol/L | 0.42(0.36–0.52) | 0.42(0.35–0.49) | 0.818 |
| L-arg ca, μmol/L | 76.4(60.8–84.7) | 57.4(46.7–74.6) | 0.023 |
| L-arg nc, μmol/L | 83.1(69.3–98.2) | 57.96(51.1–73.6) | 0.001 |
| L-arg vn, μmol/L SDA |
65.7(56.0–105.4) | 70.9(61.2–89.1) | 0.808 |
| SDMA ca, μmol/L | 0.26(0.24–0.37) | 0.34(0.28–0.38) | 0.062 |
| SDMA nc, μmol/L | 0.31(0.26–0.41) | 0.35(0.31–0.44) | 0.144 |
| SDMA vn, μmol/L | 0.44(0.36–0.52) | 0.42(0.39–0.52) | 0.903 |
| L-arg/ADMA ca | 209.07(178.01–256.74) | 174.71(144.57–233.81) | 0.746 |
| L-arg/ADMA nc | 215.14(182.15–276.11) | 169.10(130.98–206.80) | 0.275 |
| L-arg/ADMA vn | 180.82(133.93–240.17) | 182.20(133.85–206.01) | 0.387 |
| FORT ca, μmol/L | 491.69(451.81–562.08) | 447.23(358.00–684.00) | 0.112 |
| FORT nc, μmol/L | 496.92(419.77–574.08) | 486.00(329.54–580.00) | 0.695 |
| FORT vn, μmol/L | 491.85(383.31–558.73) | 450.00(358.00–684.00) | 0.918 |
| ORAC ca, μmol/L | 1.92(1.73–2.31) | 1.79(1.34–2.88) | 0.689 |
| ORAC nc, μmol/L | 2.44(1.91–2.87)) | 1.99(1.61–4.32) | 0.739 |
| ORAC vn, μmol/L | 1.91(1.59–2.24) | 2.02(1.50–3.58) | 0.641 |
ca, culprit artery; nc, non-culprit artery; vn, venous blood
Results
Our study included forty patients who were divided into 2 groups according to the median MPO level, which was 639.63ng/mL. Baseline demographic and clinical characteristics in the low and high MPO level groups are shown in Table 1. There were no statistical differences between the groups for age, baseline demographics, cardiovascular risk factors and clinical characteristics. Unrecognised non-Q-wave MI was present in the same proportion in both groups (p = 0.205). Also, there were no significant differences between the 2 groups for the hemodynamic parameters on admission. Chronic medications prior to the MI (aspirin, B-blockers) and acute pharmacological management (unfractionated heparin, low molecular weight heparin, acetylsalicylic acid, clopidogrel loading dose, abciximab infusion) were strictly identical in both groups. Furthermore, anterior wall location, median time to reperfusion, the number of diseased vessels and baseline TIMI flow in the culprit artery were similar in both groups.
MPO, MO and infarct size
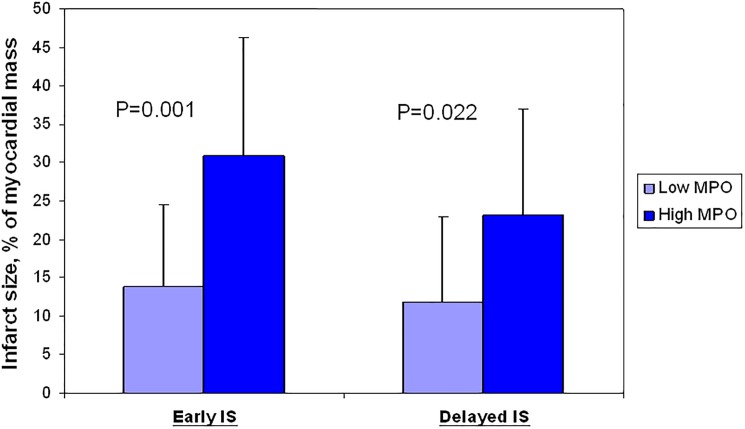
Microvascular obstruction as assessed by CMR at day 3 was observed in the same proportion (50 vs. 65%, p = 0.728) between the low vs. high MPO group levels. However, the extent of the microvascular obstruction was significantly greater in the high-MPO group (6 (0–9) vs.16.5 (0–31), p = 0.027) (Table 2), together with a greater infarct size, and a trend towards a lower left ventricular ejection fraction (LVEF) (p = 0.054) at one week. CMR data at 6 months showed that reverse systolic remodeling was two fold more present in the low-MPO group (p = 0.058). Interestingly, the extent of MO (8.5 (6.5–31) vs. 4.1 (3–11.55), p = 0.042) and IS remained significantly greater (24.5 (9.75–35) vs. 7.5 (2.5–18.75), p = 0.022) (Fig 1) in the high-MPO group. Plasma MPO levels at the culprit artery positively correlated with the IS at the acute phase but also at 6-months (respectively r = 0.497, p = 0.003 and r = 0.502, p = 0.006). Despite similar results were obtained with MPO at the ostium of the non-culprit coronary artery (r = 0.358, p = 0.038 and r = 0.454, p = 0.015), the coefficient of correlation were less good, and comparison of IS on CMR after dichotomization to the median of MPO didn’t reach the significance. Finally, MPO levels in the culprit artery appeared to correlate positively with MPO levels in both non-culprit arteries and serum, and with troponin levels and peak CK (Table 3). Multiple linear regression analysis showed that Peak CK (p = 0.012), MPO in non culprit artery (p<0.001) and the neutrophil count (p = 0.039) were independent predictors of MPO values. This statistical model can explain 44% of the variance in MPO values (R2 = 0.44) (Table 4).
Table 2. CMR data at acute phase and at 6 months.
|
Acute phase |
Low MPO Median <640 ng/mL N = 20 |
High MPO Median >640 ng/mL N = 20 |
p |
|---|---|---|---|
| LEVF, % | 56(48–60) | 49(39–54) | 0.054 |
| EDV index, mL | 88(72.5–122.5) | 95(92.5–102) | 0.294 |
| ESV index, mL | 43(27.5–59) | 52(42.5–58.5) | 0.151 |
| Presence of MO | 10(50) | 13(65) | 0.728 |
| Extent of MO, % | 6(0–9) | 16.5(0–30) | 0.027 |
| Infarct Size, % | 12(6–22) | 31(16–44) | 0.001 |
| 6-month CMR | |||
| LEVF, % | 63(55–69) | 52(44–66) | 0.064 |
| EDV index, mL | 87(76–116) | 97(85–114) | 0.315 |
| ESV index, mL | 29(25–52) | 47(28–67) | 0.109 |
| Presence of MO | 2(10) | 3(15) | 1.000 |
| Extent of MO, % | 4.1 (3–11.55) | 8.5 (6.5–31) | 0.042 |
| Infarct Size, % | 7.5(2.5–18.75) | 24.50(9.75–35) | 0.022 |
| Systolic remodeling | 6(30) | 13(65) | 0.066 |
| Reverse remodeling | 12(60) | 6(30) | 0.058 |
EDV, end-diastolic volume; ESV, end-systolic volume; IS, infarct size; LVEF, left ventricular ejection fraction; MO, microvascular obstruction.
Fig 1. Infarct size regarding MPO plasma levels in the culprit artery assessed by CMR at day 3 and 6 months.
Table 3. Determinants of the MPO value in univariate regression (n = 40).
| MPO culprit artery |
MPO non-culprit artery |
MPO serum |
||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| MPO culprit artery | *** | *** | 0.707 | <0.001 | 0.592 | <0.001 |
| MPO non-culprit artery | 0.707 | <0.001 | *** | *** | 0.633 | <0.001 |
| MPO serum | 0.592 | <0.001 | 0.633 | <0.001 | *** | *** |
| ADMA culprit artery | 0.083 | 0.639 | 0.016 | 0.929 | -0.251 | 0.159 |
| ADMA non-culprit artery | 0.054 | 0.761 | 0.170 | 0.336 | -0.225 | 0.208 |
| ADMA serum | -0.027 | 0.879 | 0.152 | 0.392 | -0.050 | 0.782 |
| SDMA culprit artery | 0.073 | 0.682 | 0.204 | 0.246 | -0.106 | 0.558 |
| SDMA non-culprit artery | 0.013 | 0.942 | 0.301 | 0.084 | -0.139 | 0.441 |
| SDMA serum | -0.190 | 0.282 | 0.023 | 0.896 | -0.224 | 0.210 |
| L-arg culprit artery | -0.181 | 0.305 | -0.457 | 0.007 | -0.611 | <0.001 |
| L-arg non-culprit artery | -0.422 | 0.013 | -0.450 | 0.008 | -0.478 | 0.005 |
| L-arg serum | -0.013 | 0.941 | -0.013 | 0.943 | -0.098 | 0.589 |
| CRP | -0.063 | 0.725 | 0.040 | 0.823 | -0.117 | 0.516 |
| Nt-proBNP | 0.172 | 0.340 | 0.220 | 0.220 | -0.019 | 0.918 |
| Creatinine clearance | 0.183 | 0.301 | 0.002 | 0.992 | 0.267 | 0.133 |
| Troponin | 0.450 | 0.008 | 0.287 | 0.107 | 0.177 | 0.325 |
| Neutrophil Count | 0.188 | 0.009 | 0.200 | 0.023 | 0.258 | 0.001 |
| Peak CK | 0.437 | 0.010 | 0.490 | 0.003 | 0.252 | 0.157 |
***NA
Table 4. Determinants of the MPO value (culprit artery) by multiple linear regression (n = 40).
| Total Population | ||
|---|---|---|
| Coefficient β | p | |
| Peak Creatinine kinase | +0.198 | 0.012 |
| MPO non culprit | +0.172 | <0.001 |
| Age | +0.133 | 0.101 |
| Neutrophil Count | +0.074 | 0.039 |
| LVEF > 40% | -0.134 | 0.265 |
| R2 | 0.44 | |
LVEF: Left Ventricular Ejection Fraction.
MPO, L-arginine and derivatives, plasma antioxidant activity assessment
Comparing the two groups, we found no significant difference for the FORT and ORAC evaluations (Table 1). Conversely, intra-coronary plasma L-arginine levels (in culprit and non-culprit arteries) were significantly lower in the high-MPO group (respectively 57.4 vs. 76.4 μmol/L; p = 0.023 and 58.0 vs. 83.1 μmol/L; p = 0.001). Interestingly, there was also a trend towards a higher plasma level of SDMA in the culprit artery in the high-MPO group (0.34 vs. 0.26 μmol/L; p = 0.062). There was no difference between these two groups regarding ADMA levels (Table 1). Nitric oxide bio-availability assessed by the L-arg/ADMA ratio at the culprit lesion site, showed that there was no difference for this parameter between the low and high-MPO group. However, patients with high MPO levels had a higher plasma HDL level (0.47 mmol/L vs. 0.38 mmol/L).
Discussion
In this study, we demonstrated an interaction between MPO levels on admission and extent of microvascular obstruction, IS and systolic LV remodeling as assessed by the gold-standard technique during acute phase, but also in the middle term follow-up. Our data highlighted the pathophysiological role of neutrophils during myocardial injury and the potential involvement of MPO in the pathophysiology of this disease, together with his consequences on the ischemic myocardium. Myeloperoxidase is a heme enzyme abundantly expressed in PMN and accounts for up to 5% of the dry weight of these cells [17]. Although MPO is also expressed in monocytes and macrophages, it has been demonstrated that 95% in the organism is PMN derived [18]. Hence MPO is considered as a valid marker of PMN activation. Therefore elevation of MPO plasma levels suggests that PMN activation is a particularly early event in the time course of AMI. Previous study [19] has shown that even when patients presented within the first 2 h after symptom onset, MPO levels were already elevated in comparison to patients with stable CAD. In contrast, however, routine markers of myocardial cell damage or coronary inflammation (i.e. hsCRP) were not increased. Funayama et al. had already proved that local MPO levels in the culprit artery may contribute to the NR phenomenon in patients with AMI [5] but did not provide evidence of mechanisms to explain their results. Furthermore, the NR was defined according to the final TIMI flow, which is far less accurate than CMR. In our study, CMR analysis provided additional information on the extent of MO, IS and ventricular remodeling, and allowed us to highlight for the first time the relationship between a high plasma level of MPO at the culprit lesion site and the enlargement of the IS after STEMI. Indeed, patients with high plasma MPO levels had a greater IS on the CMR done in the first 3 days after STEMI, and IS was still greater in these patients at 6 months after AMI. This association is strengthened by the strong correlation that we found between the troponin level, peak creatinine kinase and the plasma MPO level in the culprit artery. This was confirmed by our CMR data. In the present study, we provide evidence of 6 months persistence of microvascular obstruction in patients after reperfused AMI. Reffelmann et al. previously reported in rats that NR persists for 1 month after reperfusion and predicts worse scar thinning and infarct expansion [20]. Though we were not able to provide reliable scar thicknesses, the infarct size, expressed as a percentage of total LV mass, correlated inversely with systolic LV volumes (r-0.51, p = 0.04) [21]. Indeed, it has already been shown that plasma MPO levels were an independent predictor of myocardial infarction at two years in patients with AMI [22].
Considering the strong relation between MPO and systemic endothelial function, as a readout of vascular NO bioavailability [23,24] on the one hand and the robust data showing a markedly diminished vascular NO availability in AMI on the other hand [25,26], it might also be speculated that systemic activation of leukocytes, particularly via secretion of MPO, is one mechanism by which AMI leads to systemic endothelial dysfunction. To the best of our knowledge, our study provided new insights regarding the interaction between serum myeloperoxidase levels, endothelial dysfunction parameters (L-arginine, ADMA, SDMA) and ROS, and the impact of this interaction on clinical features including the microvascular obstruction infarct size and ventricular remodeling. First, we found that L-arginine plasma levels were significantly lower in the high-MPO group. That was unexpected, and we can hypotheses that MPO and its reactive products, including hypochlorous acid, may react with L-arginine [27], thus reducing the availability of substrate for nitric oxide synthase and forming chlorinated arginine species that inhibit the enzyme [28]. However, this is not a major reaction of HOCl as demonstrated by Pattison [29]. Myeloperoxidase and hypochlorous acid also reduce the availability of NADPH, an essential cofactor for nitric oxide synthase. Whereas we found no difference between our groups for the FORT test results, Brennan et al. provided strong data that myeloperoxidase is able to nitrate proteins via the production of reactive oxygen and nitrogen species such as peroxynitrite and nitrogen dioxide. Second, we found a trend towards higher SDMA levels in the high-MPO group: ADMA is an endogenous competitive inhibitor of all isoforms of NO synthases [30], and is now considered the most important regulator of the L-arginine/NO pathway in vivo. Our group previously reported the negative clinical impact of high admission ADMA in patients with MI [31]. Though SDMA, the stereoisomer of ADMA, is not a competitive inhibitor of NO synthases, high plasma concentrations of both ADMA and SDMA are associated with decreased brachial artery flow-mediated response. Myeloperoxidase-mediated endothelial dysfunction may be an important mechanistic link in the microvascular obstruction, since previous experimental data indicated that ADMA profoundly impairs nitric oxide synthesis in neutrophils, resulting in increased neutrophil adhesion to endothelial cells, superoxide generation, and the release of MPO. MPO appears to be a regulatory switch for ADMA bioavailability in inflammatory conditions such as MI [32]. We can hypothesize that MPO released at the culprit lesion site could destabilize culprit and non-culprit lesions and thus aggravate the microvascular obstruction.
Study limitation
This study is certainly limited by its small sample size and further studies with larger numbers of patients are required to prove our hypothesis
Conclusion
Our study provided data suggesting an interaction between MPO and the mechanisms of endothelial dysfunction. Microvascular obstruction after AMI was more severe, IS was greater and systolic LV remodeling more pronounced in the high-MPO group. Our present study may provide a new insights pathophysiology explanation for the adverse prognostic impact of NR over time.
Acknowledgments
We wish to thank Edith Fusier, Florence Bichat, Aline Chagnon, Maud Maza for their research assistance and Philip Bastable for Editorial assistance.
Data Availability
All files are available from the http://datadryad.org database (doi:10.5061/dryad.bj6c6).
Funding Statement
This work was supported by the University Hospital of Dijon, the Faculty of Medicine of Dijon, the Association de Cardiologie de Bourgogne, and by grants from the Union Régionale des Caisses d’Assurance Maladie de Bourgogne (URCAM), the Agence Regionale de Santé (ARS) de Bourgogne, the Conseil Régional de Bourgogne and the Fédération Française de Cardiologie (FFC).
References
- 1.Yunoki K, Naruko T, Komatsu R, Shirai N, Nakagawa M, Sugioka K et al. Relation of elevated levels of plasma myeloperoxidase to impaired myocardial microcirculation after reperfusion in patients with acute myocardial infarction. Am J Cardiol 2010;105:922–929. doi: 10.1016/j.amjcard.2009.11.013 [DOI] [PubMed] [Google Scholar]
- 2.Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med 2003;349:1595–604. doi: 10.1056/NEJMoa035003 [DOI] [PubMed] [Google Scholar]
- 3.Mocatta TJ, Pilbrow AP, Cameron VA, Senthilmohan R, Frampton CM, Richards AM et al. Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J Am Coll Cardiol 2007;49:1993–2000. doi: 10.1016/j.jacc.2007.02.040 [DOI] [PubMed] [Google Scholar]
- 4.Sugiyama S, Okada Y, Sukhova GK, Virmani R, Heinecke JW, Libby P. Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol 2001;158:879–91. doi: 10.1016/S0002-9440(10)64036-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Funayama H, Ishikawa SE, Sugawara Y, Kubo N, Momomura S, Kawakami M. Myeloperoxidase may contribute to the no-reflow phenomenon in patients with acute myocardial infarction. Int J Cardiol 2010;139:187–92. doi: 10.1016/j.ijcard.2008.10.018 [DOI] [PubMed] [Google Scholar]
- 6.Durante A, Camici PG. Novel insights into an "old" phenomenon: the no reflow. Int J Cardiol 2015;187:273–280. doi: 10.1016/j.ijcard.2015.03.359 [DOI] [PubMed] [Google Scholar]
- 7.Fieno DS, Kim RJ, Chen EL, Lomasney JW, Klocke FJ, Judd RM. Contrast-enhanced magnetic resonance imaging of myocardium at risk: distinction between reversible and irreversible injury throughout infarct healing. J Am Coll Cardiol 2000;36:1985–91. [DOI] [PubMed] [Google Scholar]
- 8.Lima JA, Judd RM, Bazille A, Schulman SP, Atalar E, Zerhouni EA. Regional heterogeneity of human myocardial infarcts demonstrated by contrast-enhanced MRI. Potential mechanisms. Circulation 1995;92:1117–25. [DOI] [PubMed] [Google Scholar]
- 9.Vita J.A, Brennan ML, Gokce N, Mann SA, Goormastic M, Shishehbor MH et al. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation 2004; 110: 1134–9. doi: 10.1161/01.CIR.0000140262.20831.8F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorgis L, Zeller M, Dentan G, Sicard P, Buffet P, L'Huillier I et al. RICO Survey Working Group. Prognostic value of N-terminal pro-brain natriuretic peptide in elderly people with acute myocardial infarction: prospective observational study. BMJ 2009;338:1605–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Circulation 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397 17951284 [Google Scholar]
- 12.TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. TIMI Study Group. N Engl J Med 1985; 312:932–936. doi: 10.1056/NEJM198504043121437 [DOI] [PubMed] [Google Scholar]
- 13.Lorgis L, Zeller M, Dentan G, Sicard P, Richard C, Buffet P et al. The free oxygen radicals test (FORT) to assess circulating oxidative stress in patients with acute myocardial infarction. Atherosclerosis 2010;213:616–621. doi: 10.1016/j.atherosclerosis.2010.09.018 [DOI] [PubMed] [Google Scholar]
- 14.Cao G, Alessio H.M., and Cutler R.G.. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic Biol Med 1993;14:303–311. [DOI] [PubMed] [Google Scholar]
- 15.Lorin J, Guilland JC, Korandji C, Touzery C, Bichat F, Chagnon A et al. High levels of asymmetric dimethylarginine are strongly associated with low HDL in patients with acute myocardial infarction. PLoS One 2013; 8:64796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodi V, Monmeneu JV, Ortiz-Perez JT, Lopez-Lereu MP, Bonanad C, Husser O et al. Prediction of Reverse Remodeling at Cardiac MR Imaging Soon after First ST-Segment-Elevation Myocardial Infarction: Results of a Large Prospective Registry. Radiology 2016;278(1):54–63. doi: 10.1148/radiol.2015142674 [DOI] [PubMed] [Google Scholar]
- 17.Schultz J, Kaminker K. Myeloperoxidase of the leucocyte of normal human blood. Content and localization. Arch. Biochem. Biophys 1962; 96:465–467. [DOI] [PubMed] [Google Scholar]
- 18.Shishehbor M. H.; Aviles R. J.; Brennan M. L. Fu X, Goormastic M, Pearce GL et al. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA 2003; 289: 1675–1680. doi: 10.1001/jama.289.13.1675 [DOI] [PubMed] [Google Scholar]
- 19.Goldmann BU, Rudolph V, Rudolph TK, Holle AK, Hillebrandt M, Meinertz T et al. Neutrophil activation precedes myocardial injury in patients with acute myocardial infarction. Free Radic Biol Med. 2009;47(1):79–83. doi: 10.1016/j.freeradbiomed.2009.04.004 [DOI] [PubMed] [Google Scholar]
- 20.Reffelmann T, Hale SL, Dow JS, Kloner RA. No-reflow phenomenon persists long-term after ischemia/reperfusion in the rat and predicts infarct expansion. Circulation 2003;108:2911–2917. doi: 10.1161/01.CIR.0000101917.80668.E1 [DOI] [PubMed] [Google Scholar]
- 21.Cavusoglu E, Ruwende C, Eng C, Chopra V, Yanamadala S, Clark LT et al. Usefulness of baseline plasma myeloperoxidase levels as an independent predictor of myocardial infarction at two years in patients presenting with acute coronary syndrome. Am J Cardiol 2007; 99:1364–8. doi: 10.1016/j.amjcard.2006.12.060 [DOI] [PubMed] [Google Scholar]
- 22.Bière L, Donal E, Jacquier A, Croisille P, Genée O, Christiaens L et al. A new look at left ventricular remodeling definition by cardiac imaging. Int J Cardiol 2016; 209:17–9. doi: 10.1016/j.ijcard.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 23.Eiserich JP, Baldus S, Brennan ML, et al. Myeloperoxidase, a leukocyte derived vascular NO oxidase. Science 2002; 296:2391–2394. doi: 10.1126/science.1106830 [DOI] [PubMed] [Google Scholar]
- 24.Vita J A, Brennan ML, Gokce N, et al. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation 2004; 110:1134–1139. doi: 10.1161/01.CIR.0000140262.20831.8F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amir O.; Jaffe R.; Shiran A.; Flugelman M. Y.; Halon D. A.; Lewis B. S. Brachial reactivity and extent of coronary artery disease in patients with first ST-elevation acute myocardial infarction. Am. J. Cardiol 2006; 98:754–757. doi: 10.1016/j.amjcard.2006.04.013 [DOI] [PubMed] [Google Scholar]
- 26.Karatzis EN, Ikonomidis I, Vamvakou GD, Papaioannou TG, Protogerou AD, Andreadou I et al. Long-term prognostic role of flow-mediated dilatation of the brachial artery after acute coronary syndromes without ST elevation. Am. J. Cardiol 2006; 98:1424–1428. doi: 10.1016/j.amjcard.2006.06.043 [DOI] [PubMed] [Google Scholar]
- 27.Zhang C, Patel R, Eiserich JP, Zhou F, Kelpke S, Ma W et al. Endothelial dysfunction is induced by proinflammatory oxidant hypochlorous acid. Am J Physiol Heart CircPhysiol 2001;281:1469–1475. [DOI] [PubMed] [Google Scholar]
- 28.Zhang C, Reiter C, Eiserich JP, Boersma B, Parks DA, Beckman JS et al. L-arginine chlorination products inhibit endothelial nitric oxide production. J Biol Chem 2001;276:27159–65. doi: 10.1074/jbc.M100191200 [DOI] [PubMed] [Google Scholar]
- 29.Pattison DI, Hawkins CL, Davies MJ. What are the plasma targets of the oxidant hypochlorous acid? A kinetic modeling approach. Chem Res Toxicol 2009;22:807–17. doi: 10.1021/tx800372d [DOI] [PubMed] [Google Scholar]
- 30.Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O'Hara B et al. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med 2007;13:198–203. doi: 10.1038/nm1543 [DOI] [PubMed] [Google Scholar]
- 31.Zeller M, Korandji C, Guilland JC, Sicard P, Vergely C, Lorgis L et al. Impact of asymmetric dimethylarginine on mortality after acute myocardial infarction. Arterioscler Thromb Vasc Biol 2008;28:954–960. doi: 10.1161/ATVBAHA.108.162768 [DOI] [PubMed] [Google Scholar]
- 32.Von Leitner E, Klinke A, Atzler D, Slocum JL, Lund N, Kielstein JT et al. Pathogenic cycle between the endogenous nitric oxide synthase inhibitor asymmetrical dimethylarginine and the leukocyte-derived hemoprotein myeloperoxidase. Circulation 2011;124:2735–2745. doi: 10.1161/CIRCULATIONAHA.111.060541 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All files are available from the http://datadryad.org database (doi:10.5061/dryad.bj6c6).