Figure 2.
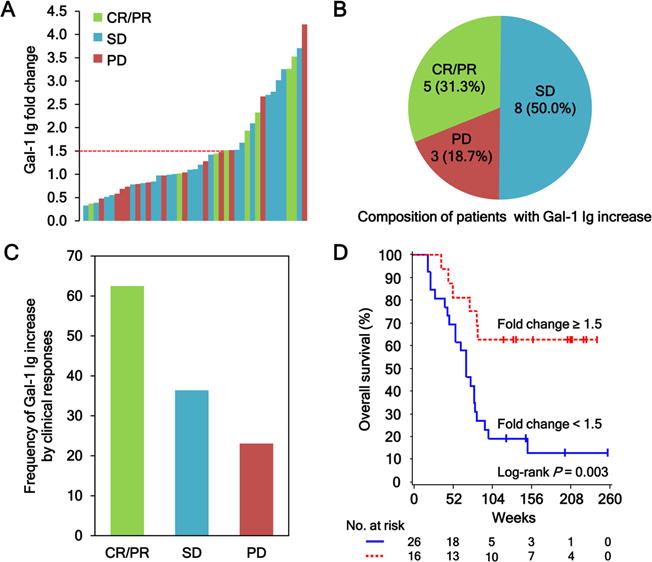
Antibody responses to Gal-1 correlate with clinical outcomes in metastatic melanoma patients treated with Ipi-Bev. A, Patients were plotted based on their Gal-1 antibody fold changes. Each bar represents a patient and the color of the bar indicates clinical response of the patient (CR, complete response; PR, partial response; SD, stable disease; and PD, progressive disease). There were 8 CR/PR (1 CR and 7 PR), 22 SD, and 13 PD patients. Gal-1 antibody titer was considered as increased when fold change ≥ 1.5. B, Composition of the 16 patients with a Gal-1 antibody increase. Numbers (and %) of CR/PR, SD and PD patients with Gal-1 antibody fold changes ≥ 1.5 are shown. C, Frequencies of Gal-1 antibody increase by clinical responses. D, Kaplan-Meier survival curves of patients based on Gal-1 antibody fold change ≥ 1.5 or < 1.5 (P = 0.0031). The median survival of the patients with Gal-1 antibody fold change < 1.5 was 70 weeks (95% CI, 47–81), whereas that of patients with fold change ≥ 1.5 was unreached.
